

Designed by Paul Smith 2006. This website is copyrighted by law.
Material contained herewith may not be used without the prior written permission of FAUNA Paraguay.
Photographs on this web-site and are used with permission.
Lutreolina crassicaudata (Desmarest 1804)
TAX: Class Mammalia; Subclass Theria; Infraclass Metatheria; Order Didelphimorphia; Family Didelphidae; Subfamily Didelphinae (Myers et al 2006). The genus Lutreolina, Thomas 1910, contains a single species. There are two recognised subspecies, that present in Paraguay is L.c.crassicaudata (Desmarest 1804) (Type Locality Asunción, Paraguay). Several other subspecies have been described on the basis of differences in size and pelage colour, but these characteristics are highly variable even within a single population and were synonymised with L.c.crassicaudata by Marshall (1978). Lutreolina is derived from the Greek meaning "otter-like", crassicaudata means "thick-tailed", describing the species most obvious physical feature. Desmarest (1804) described the species based on "Le Micouré `a grosse queue" of de Azara (1801). Synonyms adapted from Marshall (1978) and Gardner (2007).
Didelphis crassicaudata Desmarest 1804:19. Based on de Azara (1801). Type locality restricted to Asunción, Paraguay by Cabrera (1958).
Didelphys crassicaudis Illiger 1815:107. Nomen nudum.
D[idelphys]. crassicaudis Olfers, 1818:206. Type locality "Paraguay". Objective synonym.
S[arigua]. crassicaudata Muirhead 1819:429. Name combination.
Didelphis macroura Desmoulins 1824:492. Based on Azara (1801).
Peramys crassicaudata Lesson 1842:187. Name combination.
D[idelphys]. crassicaudata Schinz 1844:257. Name combination.
Didelphys mustelina Waterhouse 1846:497. Nomen nudum.
Micoureus crassicaudatus P.Gervais 1855:287. Name combination.
[Didelphys (]Metachirus[)] crassicaudatus Hensel 1872:121. Name combination.
Didelphys turneri Günther 1879:103. Type locality “Demerara”. Identified as “Better Hope, Demerara” (=Better Hope, Pomeroon-Supenaam, Guyana) by O.Thomas (1888).
Lutreolina crassicaudata O.Thomas 1910:247. New genus and name combination.
[Didelphis (Peramys)] turneri Matschie 1916:269. Name combination.
L.[utreolina] c[rassicaudata]. bonaria O.Thomas 1923:585. Type locality “Los Yngleses, Ajo” Buenos Aires, Argentina.
L.[utreolina] c[rassicaudata]. paranalis O.Thomas 1923:584. Type locality “Las Rosas, Santa Fé” Argentina.
L.[utreolina] c[rassicaudata]. lutrilla O.Thomas 1923:585. Type locality “San Lorenzo, Rio Grande do Sul” Brazil.
L.[utreolina] c[rassicaudata]. turneri O.Thomas 1923:583. First use of actual subspecific name.
Didelphis ferruginea Larrañaga 1923:346. Implied type locality Uruguay. Based in part on de Azara (1802).
Lutreolina c[rassicaudata]. travassosi Miranda-Ribeiro 1936:402. Type locality “Guariba, Estado de Säo Paulo” Brazil.
Lutreolina crassicaudus Hildebrand 1961:244. Incorrect spelling.
ENG: Thick-tailed Opossum (Marshall 1978, Redford & Eisenberg 1992), Little Water Opossum (Redford & Eisenberg 1992), Lutrine Opossum (Gardner 2007), Mink-opossum (Santori et al 2005).
ESP: Comadreja colorada (Marshall 1978, Chebez 2001, Massoia et al 2000), Comadreja colorada grande (González 2001), Coligrueso (Marshall 1978, Chebez 2001, Massoia et al 2000), Zarigüeya colorada (Chebez 2001), Cuica (Marshall 1978, Chebez 2001, Massoia et al 2000), Zarigüeya nutria (Emmons 1999), Comadreja coligruesa (Massoia et al 2006).
GUA: Mbicuré pytá (Chebez 2001), Mbicuré-pitá (Chebez 2001), Mbihkurê-pihtá (Chebez 2001), Mykure pyta (SEAM 2001), Mbicuré pythá (Parera 2002), Bechi Ac (Esquivel 2001).
DES: A medium-sized semi-aquatic opossum with a vaguely weasel-like appearance. The dense, smooth pelage is uniformly pale brownish or brownish-red dorsally, lacking any bold markings or distinguishing features and slightly paler ventrally. Pelage colour is extremely variable, and Marshall (1978) notes that captive specimens actually changed colour according to environmental conditions and diet. The pinkish rhinarium has a rounded posterior projection, sharply-defined from the furred part of the face and there is an indistinct dark patch on the snout. The ears are small, rounded with a long basal projection on the inner edge. They are barely visible above the pelage and when laid forward they reach half the distance to the eye. Eyes are dark brown. The metatragus is rounded and well-developed. Legs are short and stout. The feet are dark brown to pinkish, similarly broad with small, narrow pads on the feet and non-opposable hallux and pollex. There are five toes on each foot. The fifth hind toe reaches only to the mid-point of the first phalanx of the fourth toe. Each toe is armed with a long, slender, whitish claw. The tail is extremely thickened at the base, the junction with the body being difficult to discern. It is thickly furred along the basal half and more thinly so over the rest of its length, with the terminal 5cm of the ventral surface naked. Where thinly furred the tail is darker, almost blackish and it is white-tipped. The tail is only moderately prehensile and less so than in other Didelphids. Though the pouch is frequently reported as undeveloped, specimens examined by Lemke et al (1982) had a well-developed pouch and Parera (2002) postulates that the extent of development may in fact vary individually. There are between 9 and 11 mammae. Juveniles are similar to adults but have the tail furred along much of its length, lacking the basal swelling. CR - Cranium unique amongst the opossums on account of the long narrow zygomatic and cranial regions when compared to the short rostrum. The nasals are short and narrow, expanding posteriorly. The zygomatic arch is long and high, but robust despite the fact that it is not strongly expanded. Forehead gently domed and brain case long, narrowing in the interorbital region where smoothly rounded. (Marshall 1978). Condylobasal Length 68.7mm (54.7-82.5mm); Transverse Zygomatic Width 36.5mm (28.6-46.2mm); Temporal Constriction 7.9mm (7.2-8-8mm); Mandibular Length 53.3mm (43.8-63.7mm) (Marshall 1978). Mares & Braun (2000) give the following combined sex measurements for 2 individuals (2 males, 1 females and 1 unsexed) from Argentina: Greatest Skull Length 59.2mm (55.5-64.2mm); Condylobasal Length 58.8mm (56-63.2mm); Interorbital Constriction 7.9mm (7.5-8.4mm); Zygomatic Width 29.6mm (27.5-32.2mm); Width of Braincase 17.8mm (16.8-19.2mm); Palate Length 32.2mm (31.1-33.1mm); Length of Mandible 46.1mm (44.4-48.4mm). DF: I5/4 C1/1 P 3/3 M 4/4 = 50. Upper Tooth Row 28.9mm (22.8-31.3mm); Lower Tooth Row 28.9mm (24.1-33-8mm); Length M1-M3 10.4mm (9.5-12.2mm) (Marshall 1978). Mares & Braun (2000) give the following combined sex measurements for 2 individuals (2 males, 1 females and 1 unsexed) from Argentina: Upper Tooth Row 24.3mm (19.3-29.7mm); Lower Tooth Row 21.5mm (17.6-26-7mm). CN: 2n=22. (Redford & Eisenberg 1992). X-chromosome is metacentric and Y-chromosome acrocentric (Marshall 1978).
TRA: Not dissimilar to Didelphis sp in form and forefoot almost identical. Prints are wider than they are long with a "rotated" appearance, the digits appearing angled to the direction of the pace on the hindfoot. Hindfoot with thumb less opposable than Didelphis, though otherwise basic form is similar. (Massoia et al 2006).
MMT: A medium-sized opossum with tail approximately equal to the head and body length. There is considerable variation in size of adults, with some specimens twice the size of others, in part due to the fact that they do not reach maximum size until well after reaching sexual maturity (Marshall 1978). Males are larger than females (Eisenberg & Redford 1999). TL: 57.4cm (46.6-78.1cm); HB: 28.94cm (19.7-37.8cm); TA: 28.19cm (22.1-39cm); FT: 4.38cm (3.5-5.4cm); EA: 2.63cm (1.8-3.8cm); WT: 514.54g (176-1500g). (Redford & Eisenberg 1992, Parera 2002, Marshall 1978). Monteiro-Filho et al (2006) gives the following measurements for sexed specimens from Santa Catarina, Brazil (n= 8 males, 12 females): HB: male 29cm (+/- 3.1cm), female 25.8cm (+/- 3cm); TA: male 26.7cm (+/- 1.9cm), female 24.5cm (+/- 1.4cm); FT: male 4.1cm (+/- 0.2cm), female 3.5cm (+/- 0.2cm); EA: male 2.6cm (+/- 0.3cm), female 2.5cm (+/- 0.3cm); WT: male 435g (+/- 160g), female 358g (+/- 88g). Mares & Braun (2000) give the following combined sex measurements for 6 individuals (3 males and 3 females) from Argentina: TL: 47.18cm (37-54cm); HB: 23.27cm (19-29cm); TA: 23. 92cm (18-26cm); FT: 3.77cm (2.8-4.3cm); EA: 2.48cm (2.35-2.6cm); WT: 270.8g (176-430g).
SSP: Unlikely to be confused if seen well. Weasel-like in appearance and behaviour, it is quite unlike any other opossum. The only other opossum that is likely to take to water is the quite different Chironectes minimus. There is a vague resemblance to a small otter, but otters are considerably larger with quite different behaviour, are generally active by day and have the tail fully-furred without obvious thickening at the base.
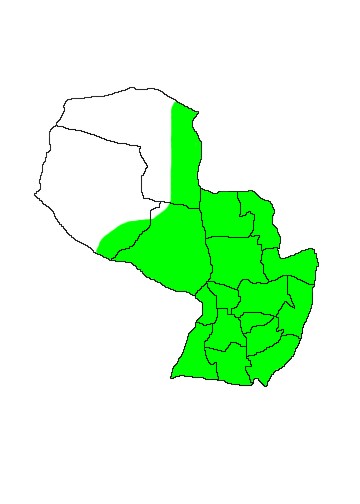
DIS: Two widely sympatric populations north and south of Amazonia assigned to two different subspecies. It has been alternatively hypothesised that the species may have historically occurred in the intervening area, or that it does still occur but has yet to be detected because of a lack of sampling (Nowak 1991). The nominate subspecies L.c.crassicaudata Desmarest 1804 is the most widespread, found over a wide area of central and southern South America from central Bolivia (not including the Amazonian regions), through eastern Paraguay (including the Pantanal region) and southern Brazil, south to Uruguay and Argentina as far as Provincia Chubut. However despite the wide geographical range it appears to be rather patchily distributed, and it may be absent from much of central Argentina, leaving the population in Provincia Jujuy somewhat isolated - animals in this region are smaller and darker than those of the rest of Argentina, though they are not currently afforded subspecific recognition (Díaz & Barquez 2002). Flores (2006) lists the species for the Provinces of Buenos Aires, Córdoba, Corrientes, Chaco, Entre Rios, Formosa, Jujuy, La Pampa, Mendoza, Misiones, Salta, Santa Fé and Tucumán in Argentina. Similarly in Brazil its precise distribution is unclear, though present in the Pantanal and the State of Rio Grande do Sul, its distribution in the region between the two is unclear. However it is not known to occur north of Rio de Janeiro in Brazil, or north of Beni in Bolivia. In Paraguay the distribution is very poorly known and there are few specimen records. The northern subspecies L.c.turneri Günther 1879, was for a long time known only from a few specimens in Guyana, though it has since been proven to occur patchily into eastern Venezuela and eastern Colombia (Nowak 1991).
HAB: Found in a variety of habitats, typically semi-open and in the vicinity of water, including natural grasslands, gallery woodland (in the Pantanal and cerrado) and palm savannas (in the Humid Chaco). At ECOSARA, San Rafael National Park it has been seen on streams in marshy areas at the intersection of pasture and Atlantic Forest (ECOSARA Bodiversity Database). In Provincia Jujuy, Argentina it occurs in dense humid forests and may do so at least marginally in other areas of the range, including Paraguay.
ALI: An aggressive predator and active hunter, exploring crevices and holes in search of prey (Parera 2002). Though best considered an opportunistic omnivore, the diet varies geographically and in some areas the species is almost entirely carnivorous, taking a variety of invertebrate and vertebrate prey, including mammals, small birds and their eggs, fish, reptiles and amphibians. Vertebrate prey is typically killed with a bite to the nape (Emmons 1999). Cáceres et al (2002) studied the diet of this species in secondary Atlantic Forest on Santa Catarina Island, Brazil by analysis of fecal samples. Of 13 fecal samples they found that crabs (54% of samples) and beetles (46%) were the main items in the diet. Other animal items documented were in order of prevalence: Opiliones (31%), Hymenoptera (23%), Lepidoptera (15%), Diptera (15%), Diplopoda (15%), Orthoptera (15%), Birds (15%), Bones (15%). Plant material included Cecropia glaziovii (85%), Piper sp. (62%), Ficus sp. (15%) and unidentified Aracaceae and Solanaceae (8% each). The species was calculated to drop a mean of 765 (+/-1995) seeds per fecal sample/night. Consumption of plant material was greatest during warm wet months (March to May) and lower or absent in cold months (June to August). The presence of undamaged seeds in fecal samples means that the species is likely an important disperser for early-colonising plants such as Cecropia. An individual at ECOSARA, San Rafael NP was observed to swim towards a singing toad Rhinella ornata,capture it and eat it at 7pm during April 2008 (David Gill pers. comm.). One stomach contained pieces of mollusc shells and sand, suggesting foraging on river bottoms. Captive individuals at New York Zoological Gardens were maintained on a diet of sliced butterfish, mixed with meat, frogs, earthworms, shrimps and mice (Davis 1966). Elsewhere captive individuals have been fed on fruit and seen to kill mammals up to the size of the wild guinea-pig Microcavia, and Massoia et al (2009) mention juveniles of Myocastor coypus and Didelphis albiventris as being taken by adults in Misiones, Argentina. Individuals have been captured in traps baited with mice (Eisenberg & Redford 1999), banana and peanut butter (Cáceres et al 2002) and are also occasionally captured in traps set for Coypu (González 2001). Astúa de Morães et al. 2003 experimentally tested the proportions of protein, lipid, carbohydrate and fibre in the diet of a single adult under laboratory condtions. Mean proportions per 100g dry weight of food were: protein 27.48g; lipid 7.20g; carbohydrate 16.28g; fibre 1.60%. Santori et al (2004) described and illustrated the gut morphology of this species and associated it with dietary habits.
REP: Little known. It would seem that they breed twice a year, once in the spring and again following the independence of the first litter. Females with young have been captured in southeastern Brazil from June to October (Monteiro-Filho et al 2006) and further south in the same country a lactating female was captured in January and recaptured in February with young (Graipel unpublished data in Monteiro-Filho et al 2006). Gestation period is about two weeks (Nowak 1991). Females are capable of conceiving even before they are dentally mature and with a mass of 200g (Monteiro-Filho et al 2006). Litter size is between 6 and 11 (Parera 2002, Eisenberg & Redford 1999) with a mean of 8.7 in southeastern Brazil (Graipel unpublished data in Monteiro-Filho et al 2006). Mares & Braun (2000) note a female with 8 young captured in October in Provincia Corrientes. The young are raised in a spherical nest of dry grass located in tree holes, amongst rushes or in burrows, either dug by the animal itself or by other species such as armadillos (Massoia et al 2000). They will even utilise bird nests. Initially the young are carried ventrally, either in a pouch or clinging to the underside of the animal, as they grow they cling to the dorsal pelage (Parera 2002). There is some evidence to suggest that males are polygynous or at least promiscuous (Monteiro-Filho et al 2006).
BEH: Activity Levels Largely nocturnal, this species is as weasel-like in behaviour as it is in appearance. The species is only occasionally active by day, but the extent to which diurnal activity is the norm and the factors influencing it have not been studied. Cáceres et al (2002) only caught the species on the ground and generally close to creeks. Locomotion It is an active, agile and efficient hunter, moving rapidly over level ground, but equally at home climbing in trees and an excellent swimmer. (Parera 2002). When swimming the species dives frequently. Santori et al (2005) found that the species swims with a quadruped, paddling gait and that whilst swimming speed was similar to terrestrial didelphids, the buoyancy and stroke frequency were closer to that of the aquatic Chironectes minimus. Under laboratory conditions mean swimming speed was 0.43m/s (+/-0.02). The dorsum, eyes and nostrils were maintained above the water surface and the nose below. Body position was roughly horizontal or slightly inclined and the body and tail made smooth bilateral movements to propel the animal through the water, with greatest propulsion provided by the hindlimbs. During the power stroke the hindlimb was forced backwards with toes extended, and on the recovery stroke the foot was swept forwards with digits adducted. Forelimbs were moved in a rotational motion slower than that of the hindlimbs and provided balance rather than propulsion. They considered that the species was not specialised for aquatic locomotion. On land they move with a trot at low speed, increasing to a gallop at high speed, but the vertebral column is never undulated. Speed during terrestrial locomotion was 1.01m/s. When walking along a horizontal tube a similar gait to low speed terrestrial locomotion was observed with a speed of 0.17 m/s. When walking along an angled trunk the speed increased to 0.35 m/s with step length 0.13m. When climbing the angled trunk only the forelimbs were used to support the animal and the hindlimbs were brought forward to form a bounding motion. When jumping the hindlimbs were brought together with the forelimbs and the animal leans forwards. The spinal column is flexed and the hindlimbs suddenly extended to generate the jumping force. Whilst in mid-air both, the entire body and limbs are extended. Home Range Little date apparently exists. In southern Brazil a recapture analysis estimated the home range of a male and a female respectively at 6517.5m2 and 8107.5m2 (Graipel unpublished data in Monteiro-Filho et al 2006). In Provincia Tucumán, Argentina home ranges of two individuals were given as 650m2 and 950m2 (Cajal 1981). Defensive Behaviour Captured animals are frequently extremely aggressive (González 2001). Enemies Recently independent juveniles fall easy prey to diurnal raptors such as Rupornis magnirostris and large owls such as Bubo virginianus and Tyto alba. Adults undoubtedly fall prey to Canids and Felids. Parasites Limardi (2006) lists the following ectoparasites from Brazil: Siphanaptera: Craneopsylla minerva (Stephanocircidae); Polygenis puelche and P.rimatus (Rhopalopsyllidae); Adoratopsylla intermedia (Ctenophthalmidae); Ctenocephalides felis (Pulicidae). Acari: Metastigmata Ixodes loricatus (Ixodidae). Two nematode species have been recorded in Argentina Travassostrongylus chacoensis and Hoineffia simplicispicula (Parera 2002).
VOC: Animals maintain contact with a high-pitched whistle. Threatened animals also whistle. (Emmons 1999). A series of postures and olfactory signals also serve purposes of communication (Parera 2002).
HUM: Currently the species does not appear to be persecuted for its fur, but in the past skins were used to make mats and as fur trimming for clothing. However the fur rapidly loses its colour and a market for the species never developed (Marshall 1978). Because of the species similarity to the Old World weasels (Mustelidae), known as "Comadrejas" in Spain, it has been hypothesised that its usage for the New World opossums (Didelphidae) originated with this species (Massoia et al 2000). The species was the subject of the children´s book "El Casamiento de la Comadreja" by Vigil (1945). In farming areas it is often persecuted for its attacks on domestic birds and their eggs, though it also plays a positive role in control rodent populations (Parera 2002).
CON: Globally considered to be of Low Risk Least Concern by the IUCN, click here to see the latest assessment of the species. The Centro de Datos de Conservación in Paraguay consider the species to be rare in Paraguay, giving it the code N3. Little-recorded because if its unobtrusive, nocturnal habits, this species does not appear to be as rare as records would suggest. In certain areas of Argentina it is even considered abundant (Parera 2002) and was considered Least Concern in that country by Chebez (2009). However, despite its presence in certain semi-urban reserves such as the Costanera Sur in Buenos Aires, it apparently does not tolerate human presence well and likely suffers from drainage associated with agriculture and the conversion of grasslands and marsh habitats into pasture through burning and other means. Olrog (1979) suggested that its increased abundance in some years in Provincia Jujuy, Argentina may be related to the abundance of sigmodontine rodents.
Citable Reference: Smith P (2008) FAUNA Paraguay Online Handbook of Paraguayan Fauna Mammal Species Account 17 Lutreolina crassicaudata.
Last Updated: 30 June 2009.
References:
Astúa de Morães D, Santori RT, Finotti R, Cerquiera R 2003 - Nutritional and Fibre Contents of Laboratory-established Diets of Neotropical Opossums (Didelphidae) p225-233 in Jones M, Dickman C, Archer M Predators with Pouches: The Biology of Carnivorous Marsupials - CSIRO Publishing, Australia.
Azara F de 1801 - Essais sur l´Histoire Naturelle des Quadrupèdes de la Province du Paraguay - Charles Pougens, Paris.
Azara F de 1802 - Apuntamientos para la Historia Natural de los Quadrúpedos del Paraguay y Rio de la Plata - La Imprenta de la Viuda de Ibarra, Madrid.
Cabrera A 1958 - Catálogo de los Mamíferos de América del Sur - Revista Museo Aregntino de Ciencias Naturales Bernadino Rivadavia Zoology 4: p1-307.
Cáceres NC, Ghizoni IR, Graipel ME 2002 - Diet of Two Marsupials Lutreolina crassicaudata and Micoureus demerarae in a Coastal Atlantic Forest Island in Brazil - Mammalia 66: p331-340.
Cajal JL 1981 - Estudios Preliminares Sobre el Área de Acción en Marsupiales (Mammalia: Marsupialia) - Physis C 40: p27-37.
Chebez JC 2001 - Fauna Misionera - LOLA, Buenos Aires.
Chebez JC 2009 - Otros que Se Van - Editorial Albatros, Buenos Aires.
Davis JA 1966 - Maverick Opossums - Animal Kingdom 69: p112-117.
Desmarest AG 1804 - Tableau Méthodique des Mammifères in Nouveau Dictionnaire d´Histoire Naturelle, Appliquée aux Arts, Principalement à l`Agriculture, à l`Économie Rurale et Domestique: Par une Société de Naturalistes et d`Agriculteurs: Avec des Figures Tirées des Trois Règnes de la Nature - Deterville Vol 24, Paris.
Desmoulins A 1824 - Didelphe in Bory de Saint-Vincent JBGM ed Dictionnaire Classique d´Histoire Naturelle - Rey et Gravier, Paris.
Eisenberg JF 1989 - Mammals of the Neotropics: Volume 1 The Northern Neotropics - University of Chicago Press, Chicago.
Eisenberg JF, Redford KH 1999 - Mammals of the Neotropics: Volume 3 The Central Neotropics - University of Chicago Press, Chicago.
Emmons LH 1999 - Mamíferos de los Bosques Húmedos de América Tropical - Editorial FAN, Santa Cruz.
Flores DA 2006 - Orden Didelphimorphia in Bárquez R, Díaz, MM, Ojeda RA eds Mamíferos de Argentina, Sistemática y Distribución - SAREM, Buenos Aires.
Gardner AL 2007 - Mammals of South America Vol 1: Marsupials, Xenarthrans, Shrews and Bats - University of Chicago Press, Chicago.
Gervais P 1855 - Histoire Naturelle de Mammifères, avec l´Indication de Leurs Moeurs, et de Leurs Rapport avec les Arts, le Commerce et l´Agriculture - L.Curmer, Paris.
González EM 2001 - Guía de Campo de los Mamíferos de Uruguay: Introducción al Estudio de los Mamíferos - Vida Silvestre, Montevideo.
Günther A 1879 - Description of a New Species of Didelphis from Demerara - Annals and Magazine of Natural History Series 5 4: p108.
Hensel R 1872 - Beiträge zue Kenntniss der Säugethiere Süd-Brasiliens - Abhandl. König. Akad. Wiss. Berlin 1872: p1-130.
Hildebrand M 1961 - Body Proportions of Didelphid (and some other) Marsupials, with Emphasis on Variability - American Journal of Anatomy 109: p239-249.
Illiger JKW 1815 - Ueberblick der Säugthiere nach Ihrer Vertheilung Über die Welttheile - Abhandl. König. Akad. Wiss. Berlin 1804-18811: p39-159.
Larrañaga DA 1923 - Escritos - Instituto Histórico y Geográfico del Uruguay, Montevideo.
Lesson RP 1842 - Nouveau tableau de règne animal. Mammifères - Arthus-Bertrand, Paris, France.
Limardi PM 2006 - Os Ectoparasitos de Marsupiais Brasileiros p27-52 in Cáceres NC, Monteiro-Filho ELA Os Marsupiais do Brasil:Biologia, Ecologia e Evolução - Editora UFMS, Campo Grande.
Mares MA, Braun JK 2000 - Systematics and Natural History of Marsupials from Argentina p23-45 in Reflections of a Naturalist: Papers Honoring Professor Eugene D Fleharty, Fort Hays Studies Special Issue 1.
Marshall LG 1978 - Lutreolina crassicaudata - Mammalian Species 91: 1-4.
Massoia E, Chebez JC, Bosso A 2006 - Los Mamíferos Silvestres de la Provincia de Misiones, Argentina - DVD-ROM.
Massoia E, Forasiepi A, Teta P 2000 - Los Marsupiales de la Argentina - LOLA, Buenos Aires.
Matschie P 1916 - Bemerkungen über die Gattung Didelphis L. - Sitzungsber. Gesells. Naturf. Freunde Berlin 1916: p259-272.
Mirando-Ribeiro A de 1936 - Didelphia ou Mammalia-Ovovipara - Revista do Museu Paulista 20: p245-424.
Monteiro-Filho ELA, Graipel ME, Cáceres N 2006 - História Natural da Cuíca-d´Água Chironectes minimus e da Cuíca-Marrom Lutreolina crassicaudata p287-295 in Cáceres N, Monteiro-Filho ELA eds Os Marsupiais do Brasil: Biologia, Ecologia e Evolução - Editorial UFMS.
Muirhead L 1819 - Mazology in Brewster D ed. The Edingburgh Encyclopedia - William Blackwood, Edinburgh.
Myers P, Espinosa R, Parr CS, Jones T, Hammond GS, Dewey A 2006 - The Animal Diversity Web (online). Accessed December 2007.
Novak RM 1991 - Walker´s Mammals of the World 5th Ed Volume 1 - Johns Hopkins, Baltimore.
Olfers I 1818 - Bemerkungen zu Illiger´s Ueberblick der Säugthiere, nach Ihrer Vertheilung über die Welttheile, Rücksichtlich der Südamerikanischen Arten p192-237 in Bertuch FI Neue Bibliothek der Wichtigsten Reisebeschreibungen zue Erweiterung der Erd - und Völkerkunde; in Verbindung mit Einigen Anderen Gelehrten Gesammelt und Herausgegeben - Verlage des Landes-Industrie-Comptoirs, Weimar.
Olrog CC 1979 - Los Mamíferos de la Selva Húmeda, Cerro Calilegua, Jujuy - Acta Zoologica Lilloana 33: p9-14.
Parera A 2002 - Los Mamíferos de la Argentina y la Región Austral de Sudamérica - Editorial El Ateneo, Buenos Aires.
Redford KH, Eisenberg JF 1992 - Mammals of the Neotropics: Volume 2 The Southern Cone - University of Chicago Press, Chicago.
Santori RT, Astúa de Moraes D, Cerqueira R 2004 - Comparative Gross Morphology of the Digestive Tract in Ten Didelphidae Marsupial Species - Mammalia 69: p27-36.
Santori R T, Rocha-Barbosa O, Vieira MV, Magnan-Neto JAB, Loguercio MFC 2005 - Aquatic, Terrestrial and Arboreal Locomotion in the Thick-tailed Opossum, Lutreolina crassicaudata (Didelphimorphia, Didelphidae) (Desmarest, 1804) - Journal of Mammalogy 86: p902-908.
Schinz HR 1844 - Systematisches Verzeichniss aller bis jetzt Bekannten Säugethiere oder Synopsis Mammalium nach dem Cuvier´schen System - Jent und Gassmann, Solothurn.
SEAM, Guyra Paraguay, PRODECHACO 2001 - Especies Silvestres del Paraguay: Guía de Identificación de Especies con Importancia Económica - PRODECHACO, Asunción.
Thomas O 1888 - Catalogue of the Marsupialia and Monotremata in the Colelction of the British Museum of Natural History - British Museum of Natural History, London.
Thomas O 1910 - A Collection of Mammals from Eastern Buenos Ayres, with Descriptions of Related New Mammals from Other Localities - Annals and Magazine of Natural History Series 8 5: p239-247.
Thomas O 1923 - The Geographical Races of Lutreolina crassicaudata - Annals and Magazine of Natural History Series 9 11: p583-585.
Vigil CC 1945 - El Casamiento de la Comadreja - Biblioteca Infantil Atlántida, Buenos Aires.
Waterhouse GR 1846 - A Natural History of the Mammalia - Hippolyte Bailliere, London.
ACKNOWLEDGEMENTS
Special thanks to Juan Carlos Chebez for providing important literature and Nilton Cáceres for very kindly reviewing texts and providing a copy of his book Os Marsupiais do Brasil.
MAP 17:
Lutreolina crassicaudata
PRINT 33:
Lutreolina crassicaudata
Adapted from Massoia et al (2006).
Click the image to enlarge it.


