

Chironectes minimus (Zimmerman 1780) Image Gallery
TAX: Class Mammalia; Subclass Theria; Infraclass Metatheria; Order Didelphimorphia; Family Didelphidae; Subfamily Didelphinae; Tribe Didelphini (Myers et al 2006). The genus Chironectes contains a single species. Chironectes is derived from the Greek meaning "hand swimmer", minimus refers to "least", the species originally being thought to be a small otter (Marshall 1978). There are four subspecies, that present in Paraguay is C.m.paraguensis (Kerr 1792) Type Locality "Rio de la Plata". In the 18th Century "La Plata" was a commonly used term for the area from Buenos Aires north to Paraguay, encompassing Uruguay and southern Brazil. Paraguay was selected as the type locality by Gardner (2007) on account of the patronymic nature of the name. Marshall (1978) used the name C.m.bresslaui Pohle 1927 for this population. The species was described in part based on the "Saricovienne" of Smellie´s (1780) translation of Buffon (1776). Mustela lutris Lacépède 1803:164 (actually Daudin in Lacépède 1802) was listed in the synonyms by Marshall (1978) as based on Buffon (1776). However Buffon´s "sarcovienne" is a composite of river and sea otters, whilst Pennant´s (1781) "sarcovienne" is clearly an opossum. Didelphys alboguttata (=Microdelphys alboguttata Burmeister 1854) has sometimes been synonymised with this species with type locality "forested regions of Brazil", though Goeldi (1894) considered it to be based on the Australian Dasyurid Dasyurus viverrinus. Synonyms adapted from Marshall (1978) and Gardner (2007):
Latra minima Zimmerman 1780:317. Type locality "Gujana". Restricted by Cabrera (1958) to Cayenne, French Guiana.
[Lutra] menina Boddaert 1784:160. Incorrect spelling.
M[ustela] (Lutra) guianensis Kerr 1792:194. Based on translation of Buffon (1776). Type locality "Cayenne", French Guiana.
M[ustela] (Lutra) paraguensis Kerr 1792:194. Based on translation of Buffon (1776). Type locality Rio de la Plata.
L[utra]. gujanensis Link 1795: 84. Based on Buffon (1776) by implication. Type locality Cayenne, French Guiana by patrimony.
Lutra saricovienna Shaw 1800: 447. Based on Buffon (1776) and Pennant (1781: 82).
[Mustela] Cayennensis Turton 1802: 58. Based on Buffon (1776). Type locality Cayenne, French Guiana.
Didelphis palmata Daudin in Lacépède 1802: 152. Based on Buffon (1776).
Lutra memia Desmarest 1803:147. Incorrect spelling.
Lutra memmina Desmarest 1804:507. Incorrect spelling.
Didelphis memmina Desmarest 1804:147. Name combination and incorrect spelling.
Chironectes variegata Illiger 1811 (1815:107). Name combination.
Chironectes variegatus Illiger 1815:107. Nomen nudum.
Didelphis lutreola Oken 1816:1134. Name unavailable.
Ch[ironectes] variegatus Olfers 1818:206. Type locality "Sudamerika".
S[arigua]. memmina Muirhead 1819:329. Name combination and incorrect spelling.
Chironectes yapock Desmarest 1820: 261. Type locality "Les bords de l´Yapock, grande rivière de Guyane" French Guyana.
Chironectes memina F.Cuvier 1825:252. Incorrect spelling and gender.
Chironectes palmata Griffith, Hamilton-Smith & Pidgeon 1827:25. Name combination.
Chironectes langsdorffi Boitard 1842: 288. Type locality near Rio de Janeiro, Brazil.
Chironectes panamensis Goldman 1914: 1. Type locality Santa Cruz de Caña, Darién, eastern Panama.
Chironectes menima bresslaui Pohle 1927: 242. Type locality "Therezopolis" Rio de Janeiero, Brazil. Incorrect spelling.
Chironectes argyrodytes Dickey 1928:15. Type locality Rio Sucio, La Libertad, El Salvador.
Chironectes minima Krumbiegel 1940:66. Incorrect gender.
Chironectes minimus Cabrera 1958:43. First use of current name combination.
ENG: Water Opossum (Gardner 2007), Yapok
ESP: Lámpara de agua (Villalba & Yanosky 2000), Comadreja aquática (Parera 2002), Zarigüeya de agua (Emmons 1999), Cuica de agua (Emmons 1999, Villalba & Yanosky 2000), Zorro de agua (Emmons 1999), Lobito overo (Massoia et al 2000), Guayquica overa (Massoia et al 2000), Gato de agua (Zetek 1930), Comadreja de agua, Perrito de agua, Guaiquica acuática, Guaiquica overa (Massoia et al 2006).
GUA: Y´apo PMA (Villalba & Yanosky 2000), Mbujá Ac (Villalba & Yanosky 2000), Joype Ac (Esquivel 2001), Yapó (Parera 2002, Emmons 1999), Yjapó, Ihapó, Yapok (Massoia et al 2006).
DES: Pelage is waterproof, dense and velvety. The top of the head and dorsum are mostly blackish with ornate greyish bands giving a "marbled impression", the throat, cheeks and dorsum are whitish. Grey "eyebrows" run from the base of the ears and may or may not join on the forehead between the eyes. The ears are pinkish basally and black on the outer half, the eyes are medium-sized and blackish. The metatragus is small and anterior basal projections are rudimentary. The chin, black nose and mouth area are naked. In addition to usual facial bristles there are long, stiff supernumerary whiskers sprouting from tufts above each eye, on the cheek below and in front of the ear and a median tuft on the chin. Rhinarium with short backward extension on upper side of muzzle. A greyish nuchal band runs across the nape and down the anterior section of the forelegs, whilst, when viewed laterally, the three broad grey lateral bands form a wide W-shape on the pelage, the hindmost running along the frontal part of the hind legs. The bands from either side of the body do not unite along the medial line of the back which is black. The pinkish forefeet lack webs, have greatly reduced claws, and possess long toes with a padded ending. The palm of the forefeet is rugose, assisting in the gripping of slippery prey, and there is a bony growth near the wrist which gives the appearance of a "sixth finger". The hind feet are larger, with the digits completely united by an interdigitary membrane. The hallux is enlarged so that the hind foot is almost symmetrical. The tail is black, furred for the basal 10%, but then naked with large scales and a small yellowish-white tip. The tail is cylindrical, tapering towards the tip and acts as a rudder when swimming - it is proportionately shorter than in most other Didelphids. A pouch is present in both sexes but only the female is able to hermetically seal the pouch. The scrotum of the male is mustard-coloured. Females in Venezuela possess four or five nipples. Juveniles are similar to adults but somewhat darker. CR - Nasals expanded posteriorly. Temporal ridges form a sagittal crest with increased age. Prominent post-orbital processes. Inter-orbital broad, flattened and with square edges. Robust zygomatic arches expand laterally. Posterior palate with single pair of vacuities opposite molars, lacking the second pair present in other Didelphids. Posterior nares narrow. Greatest Skull Length: 68.2-81mm; Zygomatic Width: 38-45.2mm; Length of Nasals: 26.3-37.5mm; Interorbital Width: 11.1-16.9mm (Marshall 1978). Mares & Braun (2000) give the following measurements for an unsexed and female Argentinian specimens respectively: Greatest Skull Length: 69.1mm, 67.1mm; Condylobasal Length: 62.6mm, 60.8mm; Zygomatic Width: 38.2mm, 38mm; Interorbital Width: 13.3mm, 12.2mm; Width of Braincase 22.1mm, 22mm; Palatal Length 41.2mm, 40.2mm; Nasal Length 28.7mm, 30.1mm; Width of Rostrum 13.3mm, 13mm; Greatest Mandibular Length 54.2mm, 51.7mm. DF: I5/4 C1/1 P 3/3 M 4/4 = 50. Length P1-M4 on Upper Row: 23-34.2mm; Length MI-M3: 10.9-13.3mm; Length of P1-M4 on Lower Row: 24.8-32mm (Marshall 1978). Mares & Braun (2000) give the following measurements for an unsexed and female Argentinian specimens respectively: Length of Upper Toothrow 30mm, 28.3mm; Length of Lower Toothrow 32mm, 39.4mm. Astúa de Morães et al. (2001) describe a supernumerary molar erupting behind the right M4 in specimen MZUSP 16545. The tooth is not fully emerged and clumped due to a lack of space on the madibular ramus, and was emerging with the crown tilted at a 45º angle CN: 2n=22. Karyotype with 10 uni-armed autosomes with terminal centromeres, an acrocentric X and a minute Y. Sex-determined by XX/XY mechanism (Marshall 1978).
TRA: Prints are distinctive on account of the unwebbed, "hand-like" forefeet in tandem with the extremely large and extensively webbed hindfeet (though webs rarely leave an impression). Forefeet have long "fingers" with an expanded ending and the first digit is short and less opposable than in other opossums. A bony tubercle at the wrist rarely leaves an impression resembling a "sixth digit" approximately perpendicular to the rest of the hand and claw marks are not usually visible. The tail is dragged behind the body and leaves a visible impression in soft mud. Prints are most frequently encountered on the muddy banks of forested streams. FP: 4.3 x 4cm; HP: 7 x 5.5cm; PA: 15cm. (Villalba & Yanosky 2000, Massoia et al 2006).
MMT: A medium-sized Didelphid with tail approximately 1 to 1.5x the head and body length. TL: 64.1cm (58-75cm); HB: 28.9cm (25-40cm); TA: 35.2cm (30-45cm); FT: 6.45cm (6-7.4cm); EA: 2.65cm (2-3.2cm); WT: 500-1300g though rarely more than 700g in the wild state (Massoia et al 2001, Parera 2002, Emmons 1999, Redford & Eisenberg 1992, Marshall 1978). Mares & Braun (2000) give the following measurements for an unsexed and female Argentinian specimens respectively: TL: 59.2cm, 63.5cm; HB: 28.2cm, 27.5cm; TA: 31cm, 36cm; FT: 6cm, 6.5cm; EA: 2.4cm, 2.7cm.
SSP: Unlikely to be confused if seen well. This is the only aquatic Didelphid and its distinctive pelage is quite unlike any related species.
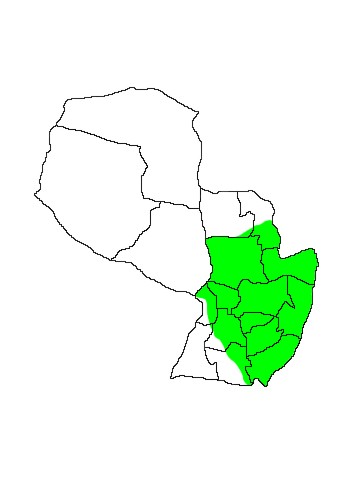
DIS: Widely distributed in the Neotropics, south from Oaxaca and Tabasco in Mexico, through South America (at elevations of up to 1800m), eastern Paraguay and extreme northern Argentina in Provincia Misiones and Corrientes. There are also recent records from northern Uruguay. Distribution is local and the species is nowhere common. Brown (2004) listed the following specimens for Paraguay: Departamento Cordillera; Salto de Pirareta, 10 km S of Piribebuy, not located (Myers, 1973, UMMZ); Departamento Paraguarí; Piribebuy (Myers, 1976, UMMZ); Parque Nacional Ybycuí (Creighton, 1979, UMMZ; Dobson, 1979, UMMZ; Myers, 1979, MZUM); Departamento Itapuá; PN San Rafael, 2 km NNW (Myers, 1978, UMMZ); Departamento Canendiyú; Curuguaty, 6.3 km N (Myers, 1976, UMMZ). Marshall recognised four subspecies based on four "loci" of distribution: C.minimus paraguensis (Kerr 1792), inhabiting northern Argentina, eastern Paraguay, southern Brazil and northern Uruguay. The remaining subspecies are C.m.minimus (Zimmerman 1780) in northeastern South America from the Guianas to the Orinoco and Amazon Basins of Brazil; C.m.panamensis (Goldman 1914) from Nicaragua south through western South America to Peru and east to Venezuela and possibly Trinidad; C.m.argyrodytes (Dickey 1928) in southern Mexico through the mountains of El Salvador to eastern Honduras.
HAB: Restricted to water courses in forested areas. It is able to colonise both sluggish and relatively fast-flowing streams provided there is sufficient prey available. It is usually absent from rivers choked with sediment (Emmons 1999). Galliez et al (2009) states that animals in the Atlantic Forest of Rio de Janeiro, Brazil were found only on fast-flowing streams with stony bottoms and preserved riparian forest.
ALI: Water Opossums swim using the hindfeet as paddles and the tail as a rudder whilst exploring the substrate with the forefeet in search of food stuffs. The forefeet are extremely nimble and they are capable of manipulating items with great dexterity. The long whiskers also act to detect potential food items whilst under water. The diet is largely carnivorous, the favoured prey appears to be slow-moving, bottom-dwelling fish (eg Silurids and Cichlids) and freshwater crustacea (Zetek 1930). These are captured by the hands or mouth and transferred to the river bank where they are consumed by the animal whilst sat on its rear legs and manipulating the prey with its forefeet. They will take invertebrates such as molluscs, aquatic insects and worms, as well as amphibians (Parera 2002). It has also been reported that they take fruits and aquatic vegetation (Hunsaker 1977) but this would seem to be unusual. Cimardi (1996) states that they are particularly fond of fish eggs during the spawning season. Captive individuals at the New York zoo thrived on slices of butterfish and a meat mix with special ingredients to assist oil production for the pelage whilst another individual lived for almost three years fed on ground bone meal and chopped raw meat lubricated with cod-liver oil (Marshall 1978). Captive adults have also been fed on mice, pink to weaned rats and chicks up to 14 days old and consumed everything including fur, feathers and extremities, though wetting of such items greatly facilitated feeding. They show agonistic behaviour when feeding. Galliez et al (2009) captured this species in Tomahawk traps placed in rivers so that the trip pan stood out of the water and baited with shrimp Litopenaeus vannamei or fish (Engraulidae). Astúa de Morães et al. 2003 experimentally tested the proportions of protein, lipid, carbohydrate and fibre in the diet of a single adult under laboratory condtions. Mean proportions per 100g dry weight of food were: protein 7.98g; lipid 0.79g; carbohydrate 7.09g; fibre 2.24%.
REP: Distribution of male territories and sex bias is conspicuous with a promiscuous or polygnynous mating system, though Galliez et al (2009) found that males were sometimes found in or near dens with female´s bearing offspring that apparently contradicts that theory. The female enters oestrus once a year but no reproductive data exists for Paraguayan populations. Captive individuals have been reported to be polyestrous. (Marshall 1978). However, during a study of wild individuals in the Atlantic Forest of Brazil, Galliez et al (2009) found that this species did not follow the normal Didelphid pattern of seasonal breeding and that breeding could take place throughout the year, with time of breeding being defined by habitat type and prey availability. Juveniles were captured in both the wet and dry seasons and their was no evidence of seasonality on recruitment. They found that sex ratio was male-biased (38 of 47 captures) and that wandering males competed for resident females. In Brazil births have been recorded in December and January, and in Argentina young have been found in August (Galliez et al 2009). Pre-copulatory behaviour involves the male circling or following the female and oral-genital contact (Marshall 1978). During copulation the male pulls the female towards him. Litters typically consist of 2 to 5 barely-developed young (mean 3.5, usually 2 or 3). Galliez et al (2009) captured two females with three young each in Rio de Janeiro, Brazil. The young make their way to the marsupium (pouch) where they complete their development faster than any other Didelphid. The female swims with the young in her pouch and it has a unique hermetic seal which prevents the developing young from drowning. However some water does enter and the pouch produces an oily solution which acts to repel water. The young also show adaptations for a low oxygen environment created when the adult is submerged. (Parera 2002). The scrotum of the male is similarly "stored" in the pouch when swimming. (Emmons 1999). Pelage begins to grow around day 22 and pigmentation appears on day 28 with colour bands appearing six days later. Eyes begin to open on day 38 and are fully open by day 43. By day 40 the young are too large to fit fully into the pouch and females nurse on their side. They leave the pouch around day 48 but return to it to suckle, sleeping alongside the female and climbing onto her back to be carried (Marshall 1978). Sexual maturity in captive individuals occurs at 10 months (Villalba & Yanosky 2000, Eisenberg & Redford 1999). The species has been successfully raised in captivity (Marshall 1978).
BEH: Activity Levels Nocturnal, solitary and unobtrusive, this is the only opossum specialised for an aquatic existence. Typically the species is encountered at night by the yellow reflection of a pair of eyes floating just above the water surface. Galliez et al (2009) stated that though the species was active throughout the night, the most intensive activity was during the first six hours after dark. Locomotion Fish (1993) documented the swimming technique of this species udner laboratory conditions. He noted that the species swam at the surface at speeds of 0.19-0.72 m/s and that the entire head and dorsum was above the water surface. The body was held close to horizontal, with a mean incline of 3.4º (+/-0.7) due to high levels of buoyancy as a result of air bubbles trapped in the velvety pelage. Propulsion is provided entirely by rhytmic, alternate, rotational paddling of the hindlimbs, the forelimbs being held outwards in front of the body with digits outstretched. Digits of the hindlimbs were extended and fully abducted during the power strokes and adducted during the recovery stroke. The recovery phase was 1.8x as long as the power phase. On land the back is noticeably curved, and curiously the animals retain the custom of feeling with the forefeet as they walk. Captive individuals have been observed to climb and to jump for distances of 60cm, but the tail is prehensile but is too thick to be of effective use when climbing (Nowak 1991). Home Range Galliez et al (2009) calculated home ranges of adults in the Atlantic Forest of Rio de Janeiro de vary between 844 and 3742m of stream with a population density of 0 to 1.34 individuals/km of river. Males maintained territories up to 4x larger than those of females and there was overlap in both male-male and male-female territories. Males had between 19-41.2% of their home range overlapped by females, and females between 44.2 and 49.3% of their home range overlapped by males. Roosts Water Opossums spend the day in small riverside caves, built close to the water level and with an entrance diameter of 10-12cm (Parera 2002). Nests may be holes in riverbanks, under tree roots or rarely on the surface. (Emmons 1999). Of 21 nests located by Galliez et al (2009), 15 were at the river margin with an entrance formed by stones and tree roots, 3 in holes at the river margin formed only by tree roots, and 3 formed by tree roots but away from the river margin. A single male was found to use as many as seven different dens, though favoured two which were used on 63.7% of occasions. One excavated nest in Panama had a tunnel of 0.6m in length descending at a 45º angle and terminating in a nest chamber (Zetek 1930). The nest cavity is lined with leaves and other plant material and adults may even bite off grass stems specifically for this purpose. Individuals have been seen to transport such material by pushing it under the body with their forefeet and holding it with their tails (Redford & Eisenberg 1992, Marshall 1978). One ground nest occupied by a male was found close to a stream bank with a diameter of 15cm and lined with dry leaves. When disturbed the occupying male dived, emerging on the other side of the stream and entering a hole in the bank (Marshall 1978). Captive individuals did not defecate in the nest chamber, but nor did they attempt to remove soiled materials. Zetek (1930) reports that a nest found in Panam was littered with remains of crustaceans. Defensive Behaviour Captured individuals can be aggressive, periodically opening and snapping the jaws and animals at the Lincoln Park Zoo attempted to bite when handled (Marshall 1978). Zetek (1930) reports that a captured individual in Panama hunched itself as if to jump when approached and hissed and snapped the jaws, but by the fourth day of captivity allowed itself to be handled. Enemies No data is available for Paraguay. In Argentina it has been noted in the diet of large eagles such as Hawk-eagles Spizaetus, but these eagles are rare in Paraguay and likely have very limited impact on the population of the species. Felines such as Ocelot, Jaguar and Puma would also be likely to take this species. (Parera 2002). Parasites Arthropods (Doloisia, Rhopalias, Stenopsylla, Tritopsylla); Tapeworms (Ligula, Sparganum); Flukes (Amphimerus). (Marshall 1978). Limardi (2006) listed the flea Siphanoptera Adoratopsylla sinnuata (Ctenophthalmidae) for Brazil. Thatcher (2006) noted the Trematoda Rhopalias baculifer (Rhopaliasidae) for Brazil. Longevity The longevity record for a captive individual is 2 years and 11 months (Nowak 1991, Marshall 1978).
VOC: Emits dry screeches when threatened (Emmons 1999).
HUM: There are no known human uses of this species in Paraguay. It does not appear to be hunted for food and despite its attractive pelage it has only recently been exploited for the fur trade in Peru (Nowak 1991). Its preference for forested habitats and specialised mode of life mean that it has little contact with humans.
CON: Globally considered to be of Low Risk Near Threatened by the IUCN, click here to see the latest assessment of the species. The Centro de Datos de Conservación in Paraguay consider the species to be rare in Paraguay, giving it the code N3. The species is local and rarely observed, yet its secretive, nocturnal habits afford it some protection. The major threats appear to be habitat destruction, water pollution as a result of pesticide run-off from agriculture, introduction of exotic species and the continuing trend for damming projects which are consistently and irreversibly altering the river systems of Paraguay. Currently there has been no commercial exploitation of its pelage but any systematic persecution of the species would likely have catastrophic effects on its population. Galliez et al (2009) suggested that this species is sensitive to changes in river microhabitat and might be a useful indicator species for changes in river status. Flores (2006) considers the species potentially vulnerable in Argentina as a result of persecution, though Chebez (2009) disagrees that the species is persecuted and considers the species amply distributed at low numbers in Misiones. He suggests that it should be considered Least Concern in Argentina, though acknowledges that little data is available.
Citable Reference: Smith P (2007) FAUNA Paraguay Online Handbook of Paraguayan Fauna Mammal Species Account 4 Chironectes minimus.
Last Updated: 30 June 2009.
References:
Astúa de Morães D, Lemos B, Finotti R, Cerquiera R 2001 - Supernumerary Molars in Neotropical Opossums (Didelphimorphia, Didelphidae) - Mammalian Biology 66: p193-203.
Astúa de Morães D, Santori RT, Finotti R, Cerquiera R 2003 - Nutritional and Fibre Contents of Laboratory-established Diets of Neotropical Opossums (Didelphidae) p225-233 in Jones M, Dickman C, Archer M Predators with Pouches: The Biology of Carnivorous Marsupials - CSIRO Publishing, Australia.
Burmeister H 1854 Systematische Uebersicht der Thiere Brasiliens: Welche während einer Reise durch die Provinzen von Rio de Janeiro und Minas Geraës Gesammlt oder beobachtet wurden Vol 1 - G.Reimer, Berlin.
Boddaert P 1784 - Elenchus Animalium - Rotterdam Vol 2.
Boitard P 1845 - Les Jardines des Plantes - JJ Dubochet et Cie, Paris.
Brown BE 2004 - Atlas of New World Marsupials - Fieldiana Zoology 102.
Buffon G 1776 - Histoire Naturelle, Générale et Particulière Avec la Description du Cabinet du Roi - Paris Suppl Vol 3.
Cabrera A 1958 - Catálogo de los Mamíferos de América del Sur - Revista Museo Aregntino de Ciencias Naturales Bernadino Rivadavia Zoology 4: p1-307.
Cartés JL 2007 - Patrones de Uso de los Mamíferos del Paraguay: Importancia Sociocultural y Económica p167-186 in Biodiversidad del Paraguay: Una Aproximación a sus Realidades - Fundación Moises Bertoni, Asunción.
Chebez JC 2009 - Otros que Se Van - Editorial Albatros, Buenos Aires.
Cimardi AV 1996 - Mamíferos de Santa Catarina - FATMA, Florianópolis.
Cuvier F 1825 - Des Dents de Mammifères, Considérées Comme Caractères Zoologiques - FG Levrault, Strasbourg & Paris.
Desmarest AG 1803 - Sarigue ou Didelphe (Didelphis), Genre de Quadrupèdes de l´Ordire des Carnassiers, Sous-odre des Pédimanes in Nouveau Dictionnaire d´Histoire Naturelle, Appliquée aux Arts, Principalement à l`Agriculture, à l`Économie Rurale et Domestique: Par une Société de Naturalistes et d`Agriculteurs: Avec des Figures Tirées des Trois Règnes de la Nature - Deterville Vol 20, Paris
Desmarest AG 1804 - Tableau Méthodique des Mammifères in Nouveau Dictionnaire d´Histoire Naturelle, Appliquée aux Arts, Principalement à l`Agriculture, à l`Économie Rurale et Domestique: Par une Société de Naturalistes et d`Agriculteurs: Avec des Figures Tirées des Trois Règnes de la Nature - Deterville Vol 24, Paris.
Desmarest AG 1820 - Mammalogie ou Description des Espèces de Mammifères - Paris Vol 1.
Dickey D 1928 - A New Marsupial from El Salvador - Proceedings of Biological Society of Washington 41: p15-16.
Eisenberg JF 1989 - Mammals of the Neotropics: Volume 1 The Northern Neotropics - University of Chicago Press, Chicago.
Eisenberg JF, Redford KH 1999 - Mammals of the Neotropics: Volume 3 The Central Neotropics - University of Chicago Press, Chicago.
Emmons LH 1999 - Mamíferos de los Bosques Húmedos de América Tropical - Editorial FAN, Santa Cruz.
Esquivel E 2001 - Mamíferos de la Reserva Natural del Bosque Mbaracayú, Paraguay - Fundación Moises Bertoni, Asunción.
Fish FE 1993 - Comparison of Swimming Kinematics Between Terrestrial and Semiaquatic Opossums - Journal of Mammalogy 74: p275-284.
Flores DA 2006 - Orden Didelphimorphia in Bárquez R, Díaz, MM, Ojeda RA eds Mamíferos de Argentina, Sistemática y Distribución - SAREM, Buenos Aires.
Galliez M, Souza Leite M de, Quieroz TL, dos Santos Fernandez FA 2009 - Ecology of the Water Opossum Chironectes minimus in Atlantic Forest Streams of Southeastern Brazil - Journal of Mammalogy 90: p93-103.
Gardner AL 2007 - Mammals of South America Volume 1: Marsupials, Xenarthrans, Shrews and Bats - University of Chicago Press.
Goeldi EA 1894 - Critical Gleanings on the Didelphyidae of the Serra dos Orgãos, Brazil - Proceedings of the Zoological Society of London 1894: p457-467.
Goldman EA 1914 - Descriptions of Five New Mammals from Panama - Smithsonian Miscellaneous Coll. 63: p1-7.
Griffith E, Hamilton-Smith C, Pidgeon E 1827 - The Class Mammalia Arranged by Baron Cuvier with Specific Descriptions in Griffith et al The Animal Kingdom Arranged in Conformity with its Organization by the Baron Cuvier .... with Additional Descriptions of all the Species Hitherto Named and Many Not Before Noticed - Geo. B. Whittaker, London.
Hunsaker D 1977 - The Biology of Marsupials - Academic Press, New York.
Iliger C 1811 - Prodromus Systematis Mammalium et Avium Additis Terminis Zoographicis Utriudque Classis - C. Salfeld, Berlin.
Illiger JKW 1815 - Ueberblick der Säugthiere nach Ihrer Vertheilung Über die Welttheile - Abhandl. König. Akad. Wiss. Berlin 1804-18811: p39-159.
Kerr R 1792 - The Animal Kingdom or Zoological System of the Celebrated Sir Charles Linnaeus - London.
Krumbiegel I 1940 - Die Säugthiere der Südamerika Expeditionen Prof. Dr. Kriegs 5. Schwimmbeutler. Zool. Anz. 132: p63-72.
Lacépède BGE 1803 - Tableau des Divisions, Sous-divisions, Ordres et Genres des Mammifères in Buffon GLL 1799 de Histoire Naturelle, Paris.
Link HF 1795 - Beschreibung der Naturaliensammling der Universität zu Rostock 2: p84.
Mares MA, Braun JK 2000 - Systematics and Natural History of Marsupials from Argentina p23-45 in Reflections of a Naturalist: Papers Honoring Professor Eugene D Fleharty, Fort Hays Studies Special Issue 1.
Massoia E, Chebez JC, Bosso A 2006 - Los Mamíferos Silvestres de la Provincia de Misiones, Argentina - DVD-ROM.
Massoia E, Forasiepi A, Teta P 2000 - Los Marsupiales de la Argentina - LOLA, Buenos Aires.
Marshall LG 1978 - Chironectes minimus - Mammalian Species 109: 1-6.
Monteiro-Filho ELA, Graipel ME, Cáceres N 2006 - História Natural da Cuíca-d´Água Chironectes minimus e da Cuíca-Marrom Lutreolina crassicaudata p287-295 in Cáceres N, Monteiro-Filho ELA eds Os Marsupiais do Brasil: Biologia, Ecologia e Evolução - Editorial UFMS.
Myers P, Espinosa R, Parr CS, Jones T, Hammond GS, Dewey A 2006 - The Animal Diversity Web (online). Accessed December 2007.
Novak RM 1991 - Walker´s Mammals of the World 5th Ed Volume 1 - Johns Hopkins, Baltimore.
Oken L 1816 - Lehrbuch der Naturgeschichte - Dritter Theil. Zoologie, August Schmid und Comp.
Olfers I 1818 - Bemerkungen zu Illiger´s Ueberblick der Säugthiere, nach Ihrer Vertheilung über die Welttheile, Rücksichtlich der Südamerikanischen Arten p192-237 in Bertuch FI Neue Bibliothek der Wichtigsten Reisebeschreibungen zue Erweiterung der Erd - und Völkerkunde; in Verbindung mit Einigen Anderen Gelehrten Gesammelt und Herausgegeben - Verlage des Landes-Industrie-Comptoirs, Weimar.
Parera A 2002 - Los Mamíferos de la Argentina y la Región Austral de Sudamérica - Editorial El Ateneo, Buenos Aires.
Pennant T 1781 - History of Quadrupeds - B.White, London.
Redford KH, Eisenberg JF 1992 - Mammals of the Neotropics: Volume 2 The Southern Cone - University of Chicago Press, Chicago.
SEAM, Guyra Paraguay, PRODECHACO 2001 - Especies Silvestres del Paraguay: Guía de Identificación de Especies con Importancia Económica - PRODECHACO, Asunción.
Shaw G 1800 - General Zoology or Systematic Natural History - G.Kearsley London 1(2): p447.
Turton W 1802 - A General System of Nature Through the Three Grand Kingdoms - Trans. Of C. von Linné Systema Naturae 1: p58.
Villalba R, Yanosky A 2000 - Guía de Huellas y Señales: Fauna Paraguaya - Fundación Moises Bertoni, Asunción.
Zimmermann E 1780- Geographische Geschichte des Menschen und der Allgemein Verbreiten Vierfüssigen Tiere 1-3 Leipzig Vol 2.
Zetek J 1930 - The Water Opossum Chironectes panamensis Goldman - Journal of Mammalogy 11: p470-471.
ACKNOWLEDGEMENTS
Special thanks to Juan Carlos Chebez for providing important literature and Nilton Cáceres for very kindly reviewing texts and providing a copy of his book Os Marsupiais do Brasil.
MAP 4:
Chironectes minimus
PRINT 4:
Chironectes minimus
Adapted from Massoia et al (2006) and Yanosky & Villalba (2000).
Click the image to enlarge it.
Designed by Paul Smith 2006. This website is copyrighted by law.
Material contained herewith may not be used without the prior written permission of FAUNA Paraguay.
Photographs on this web-site and are used with permission.


