

Designed by Paul Smith 2006. This website is copyrighted by law.
Material contained herewith may not be used without the prior written permission of FAUNA Paraguay.
Photographs on this web-site were taken by Paul Smith, Hemme Batjes, Regis Nossent, Frank Fragano,
Alberto Esquivel, Arne Lesterhuis, José Luis Cartes, Rebecca Zarza and Hugo del Castillo and are used with their permission.
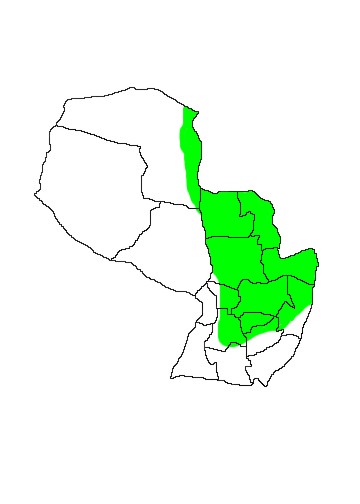
MAP 9: Philander opossum
Philander opossum (Linnaeus 1758) Image Gallery
TAX: Class Mammalia; Subclass Theria; Infraclass Metatheria; Order Didelphimorphia; Family Didelphidae; Subfamily Didelphinae (Myers et al 2006). Six species are recognised in this genus (Patton & da Silva 1997, Lew 2006), one is present in Paraguay. The scientific name is derived from the first vernacular name "Philander opossum, sive Carigueja" used by Seba (1734). The genus Philander was defined by Brisson in 1762, though it is often credited to Tiedemann 1808. However Tiedemann clearly used the name when referring to the Didelphis of Linnaeus (Allen 1900). In 1998 the International Commission on Zoological Nomenclature determined that Brisson´s name had priority over that of Tiedemann. There has been considerable debate as to which generic name should take precedence for this species with Pine (1973) arguing that Metachirops Matschie 1916 is the correct designation for this species and that Philander should apply to the Brown Four-eyed Opossum here referred to Metachirus Burmeister 1854. However his arguments failed to gain widespread support and Philander is the most widely accepted generic name for this species (Nowak 1991, Redford & Eisenberg 1992, Emmons 1999). Based on mtDNA cytochrome-b gene sequences and DNA-DNA hybridisation data Philander is the sister group of Didelphis. (Castro-Arellano et al. 2000).
The species limits in Philander are far from resolved and it is likely that more species will be described in future. Currently the distribution of the subspecies attributed to P.opossum, and even the names referable to the populations are unclear and much work remains to be done to clarify the situation. The two most recent detailled reviews of the species disagree considerably as to the distribution and nomenclature of the subspecies involved. In terms of the southern populations Hershkovitz (1997) attributes the southern Brazilian, Paraguayan, Bolivian and eastern Peruvian populations to P.o.quica Temminck 1824, confining P.o.frenata Olfers 1818 (now considered a distinct species P.frenata) to Salvador, Bahía and maps the nominate P.o.opossum for the entire Amazon population. Castro-Arellano et al (2000) however applied P.o.frenatus (sic) to the coastal Atlantic forest subspecies extending from Bahía State in Brazil to Provincia Misiones, Argentina, with P.o.azaricus used for the Paraguayan population and animals inhabiting the Pantanal and cerrado of Brazil, whilst those of Bolivia, western Amazonian Brazil and eastern Peru were attribted to P.o.canus and the nominate P.o.opossum to animals in the eastern Amazon, Guyanas and eastern Venezuela. Massoia et al (2000) attribute the northern Argentinean population to P.o.azaricus in agreement with Patton & da Silva (1997) who consider P.frenata a distinct species with an entirely Brazilian range that extends from at least the State of Paraná north to Bahía and inland to Minais Gerais and Goias. However they note that the relationship of P.frenata to P.o.azarica (sic) remains to be determined and maintained azarica within P.opossum without any further justification other than the fact that it is traditionally placed there. The two "unnamed subspecies" mentioned in Hershkovitz (1997) and Castro-Arellano et al (2000) were later defined as separate species, Philander deltae and Philander monodolfi by Lew et al (2006). Here we follow the subspecies as defined by Castro-Arellano et al (2000), modified slightly according to other published data and incorporating the updates provided by Lew et al (2006). We recognise the limitations of this arrangement however and note that the specific and subspecific designation of the Paraguayan population remains to be confirmed.
[Didelphis] opossum Linnaeus 1758:55 Type Locality Paramaribo, Surinam.
Didelphis opossum Brongniart 1792:115 Incorrect emendation.
Philander virginianus Tiedemann 1808:426 New name for D.opossum Linnaeus.
S[arigua] opossum Muirhead 1819:429 New name combination.
D[idelphys] opossum Wagner 1843:44 Incorrect emendation.
[Didelphis ([Metachirus]) opossum ] Burmeister 1865:69 New name combination.
Gamba opossum Liais 1872:329 New name combination.
Metachirus fuscogriseus pallidus Allen 1901:215 Type Locality Orizaba, Veracruz, Mexico.
[Didelphis (Metachirops)] pallidus Matschie 1961:268 New name combination.
[Didelphis (Metachirops)] opossum Matschie 1961:268 New name combination.
Didelphis austro-americana Thomas 1923:604 Type Locality "Suriname". Name preoccupied.
Metachirops opossum pallidus Miller 1924:7 New name combination.
Metachirops opossum Tate 1939:161 New name combination.
Philander opossum Gilmore 1941:316 New name combination based on Brisson 1762 not Tiedemann 1808.
Philander opossum pallidus Dalquest 1950:2 New name combination.
ENG: Grey Four-eyed Opossum
ESP: Comadreja de cuatro ojos (Massoia et al 2000), Comadreja de anteojos (Massoia et al 2000) (Emmons 1999, Esquivel 2001), Guayquica overa (Massoia et al 2000, Parera 2002), Mantequera (Massoia et al 2000), Chucha (Massoia et al 2000, Redford & Eisenberg 1992), Cuica común (Massoia et al 2000), Zorro de cuatrojos (Redford & Eisenberg 1992), Zarigüeya de cuatro ojos grís común (Emmons 1991), Carachupa cuatro ojos (Cuéllar & Noss 2003).
GUA: Guaikí (Massoia et al 2000, Redford & Eisenberg 1992).
DES: Slender with a large head and elongated, conical rostrum. Pelage short, dense and smooth, each hair pale-based with a darker central band and silvery-tip. Under ultra-violet light the pelage is fluorescent purple and pink. The dorsum is greyish or greyish-brown, becoming paler and whiter towards the flanks and creamy-white on the throat, cheeks and venter. The head is uniform with the body with conspicuous white patches above the eyes (the "spectacles") and less obvious, smaller patches at the anterior base of the ears. The ears are large, rounded and pinkish with blackish borders to the pinnae. The nose, upper lip and feet are naked and pinkish in colour. The prehensile tail is long, furred for the first 5-8cm (approximately 17% of its total length) and greyish at the base with a sharply-demarcated white tip. It is cylindrical, slender, scaly and tapers towards the tip. Hind feet have opposable pollex and hallux, modified for grasping. The maruspium, which opens from the side, is orange in females that have had young. The scrotum of the male is black and breeding males develop a small yellowish patch on the sides in front of the thighs. Females possess between 5 and 9 mammae concealed within the marsupium, usually 7, arranged in two rows of three with a median teat between them. Juveniles are similar to adults with have finer, softer fur. CR - Skull is narrow and slender. Philander has a relatively large brain in comparison with other Didelphids (Redford & Eisenberg 1992). Bony palatte with four fenestrae. Sagittal and occipital crests are well-developed in adults and auditory bullae are small. Condylobasal Length: 60-82.1mm; Zygomatic Width: 28-43.7m; Braincase Width: 18-24mm; Palate Length: 34.1-50.2mm (Castro-Arellano et al. 2000). DF: I5/4 C1/1 P 3/3 M 4/4 = 50. First incisor largest on upper row, absent on lower mandible. Upper canine long, slender and decurved, lower canine similar but smaller. Premolars unicuspid with two roots. Upper P1 about half the size of P2, and P3 deciduous. Young at weaning lack molars, the molars then begin to appear in sequence. Upper M1 longer and larger than M2. Upper M4 about half the size of M3. Lower M2 or M3 largest, lower M4 smallest. The appearance of M4 is accompanied by the loss of P3. Dentition is complete at 1 year of age. From this point on age can be determined by tooth wear. Body mass continues to increase after full eruption of molars (Hershkovitz 1997). CN: 2n=22. FN=20. Karyotype with 10 uni-armed autosomes with terminal centromeres, an acrocentric X and a minute Y (Castro-Arellano et al. 2000).
TRA: No information.
MMT: A large and robust, cat-like Didelphid, with tail slightly longer than the head and body. Males initially the same size of females, but outgrow them by maturity. Captive individuals may reach much larger sizes than wild individuals, and largest wild individuals are not necessarily the oldest. Dental size, fixed at eruption, is the true indicator of body size (Hershkovitz 1997). TL: 54.21cm (43.7-62cm); HB: 26.56cm (20.2-39cm); TA: 28.54cm (19.5-40.5cm); FT: 4.2cm (2.9-5.2cm); EA: 3.52cm (1.8-4.3cm); WT: 444.4g (200-960g) males heavier than females. In captivity may reach 1500g; WN: 9g. (Massoia et al 2001, Emmons 1999, Redford & Eisenberg 1992, Castro-Arellano et al. 2000).
SSP: The only other species to share the "eyes" of the Grey Four-eyed Opossum is the Brown Four-eyed Opossum, which unsurprisingly is most easily distinguished by its brownish pelage. When viewed frontally note that the white spots above the eyes are smaller and more widely-spaced in Brown FEO. The second pair of "eyes" are much reduced and located in front of the ears, those of the Brown FEO being extensive and located behind the ears. The tail of the Grey FEO is proportionately shorter and furred at the base for the first 6 to 8 cm, as well as being bicoloured with a clearly demarcated tip - that of the Brown FEO is naked to the base and becomes gradually paler along its length. Structurally the snout is more pointed and the legs are longer in Brown FEO. Female Brown FEO do not possess a pouch.
DIS: Widely distributed from sea-level to 1,650m from southern Mexico to extreme northern Argentina where it has been collected in the Chaco and Provincias of Formosa and Misiones. P.o.azaricus occurs in Paraguay, northern Argentina and southwestern Brazil. In Paraguay it is scantily distributed through eastern Paraguay and the eastern part of Departamento Alto Paraná, the Paraguayan Pantanal. It is not listed for the Mbaracayú Biosphere Reserve (Esquivel 2001) and Lowen et al (1996) did not record it during extensive surveys of the Atlantic Forests of eastern Paraguay in 1992 and 1995, nor did they interview locals who were familiar with the species. Brown (2004) listed the following specimens for Paraguay: Departamento Concepción; Rio Aquidaban, Paso Horqueta (Creighton,1979, UMMZ); Aca Poi (Wharton, 1950, USNM); Departamento Cordillera; Tobati, 12 km N (Myers, 1973, MVZ); Departamento Central; Luque, 17 km E (Koford, 1972, MVZ; Myers, 1976, UMMZ). The remaining subspecies are as follows: P.o.opossum western Venezuela, the Guyanas and Amazonian northern and central Brazil; P.o.canus northern and central Bolivia, eastern Peru and northwestern Brazil; P.o.melanurus Pacific slope of Ecuador and Colombia; P.o.fuscogriseus Central America from southern Mexico to Panama. See taxonomic note above.
HAB: Adaptable and able to colonise a variety of habitats with the preference for forested areas close to rivers or swamps. In Brazil they were found most commonly in humid areas, but occur in almost all vegetation types (Redford & Eisenberg 1992). Typically they occur in humid and semi-humid evergreen forests, such as the Atlantic Forest and the seasonally-inundated forests of the Pantanal. The water balance of Philander suggests that it is an obligate inhabitant of mesic environments (Fonseca & Cerqueira 1991).
ALI: Omnivorous but primarily carnivorous, taking mainly invertebrates and small vertebrates supplemented with fruit. Nowak (1991) lists small mammals, birds and their eggs, reptiles, amphibians, insects, freshwater crustacea, snails, earthworms, fruit and carrion as dietary items. The species has also been observed to raid dust-bins and to kill poultry. It forages both on the ground in dense undergrowth near rivers and at mid-levels of the forest canopy, actively pursuing prey and investigating potential hiding places under fallen trunks and in rotten wood. It pursues frogs, following their call to the source. In French Guiana stomachs were found to contain 85% animal matter and 15% fruit and seeds. In Mexico the three most important items identified in scats over the course of a year were Scarabaeid beetles (in 74% of scats), crustacea (67%) and Cecropia seeds (60%). The only vertebrates recorded were birds which occurred in just 3% of scats. Plant matter was shown to be more prominent in the wet season and they have been reported to open healed wounds in trees to feed on sap. Of fruits included in the diet 77% were less than 50mm in diameter and typically involved species with a high water content, fleshy pulp, high sugar or lipid content and low nitrogen. Fruit is apparently important for lactating females as the extra sugars help cover the increased energy demand during this period, with a preference shown for mature fruit that has fallen to the ground. The species is an important disperser for colonising species such as Cecropia as it drops seeds in open secondary growth areas. In captivity the species is said to be more carnivorous than other Didelphids and animals have been trapped using flesh as bait. In fact they have even been seen to raid rodent traps for their contents and mist nets to take bats. (Redford & Eisenberg 1992, Castro-Arellano et al. 2000). Hershkovitz (1997) noted that a captive female ate "everything served" including beef, insects, chicken legs, peanut butter and mice. Mice were attacked instantly, the head and neck being crunched and the entire animal being swallowed. Though food was provided during the day most was consumed at night, though water was taken throughout the day. However if a live insect or mouse was provided during the day it would be instantly attacked. Cannibalism has been reported in captive individuals, with weaned young being eaten by the mother and by each other (Charles-Dominique 1983).
REP: Breeding occurs from August to February in Misiones, Argentina and Rio de Janeiro, Brazil. In other parts of their range they may breed all year round eg Vera Cruz, Mexico. Females with pouched young have been found in Nicaragua from February to October, in Panama from April to July and Colombia in June, September and October (Nowak 1991). Males are sexually active throughout the year but the testes vary seasonally in size, though not in mass or spermatogenesis. Fleming (1973) states that the species is seasonally polyestrous in Panama and two or more litters may be produced by each female from January to November. Breeding is affected by the rains and provision of food - females may abort a brood by stopping lactation if there is insufficient food available to her. Success rates were as high as 97% for the first two litters in French Guyana, but dropped to just 27% for third annual litters which conincided with a period of food scarcity (Charles-Dominique et al 1981). Litter size appears to be larger in the northern parts of the range but overall the litter is smaller on average than that of Didelphis. Average litter size of 21 females in Nicaragua was 6 (range 3 to 7), whilst in Surinam there was an average of 2.8 pouched young in six examined specimens (range 1 to 5). In southeastern Brazil 4.5 was the average litter size amongst seven females whilst in northeastern Argentina the observed range of litter size is from 4 to 6. A litter from Paraguay photographed by José Luis Cartés contained 5 young. The gestation period is from 13 to 30 days (Cimardi 1996). Ovarian cycling is suspended during lactation but not during gestation, the act of suckling being key to the suspension of the cycle. Pouched young measure 35-105mm (the latter figure for a captive individual). In Mexico 44% of litters were male-biased, whereas in Panama and Nicaragua the average ratio was very close to 1:1. In Brazil a litter with 4 males and 1 female young was found. Juveniles are weaned at 68-75 days when they weigh 50-75g (100-200g for captive individuals). The nest phase lasts as little as 8 to 15 days before dispersal of the young, with members of the litter becoming more anti-social towards each other following weaning.. Survival chances of a litter is greatly affected by the age of the mother. Females <11 months old and >17 months old have the highest mortality rate of offspring. Males reach sexual maturity rapidly at 7 months, and females are sexually mature by 6 or 7 months, though the first oestrus is not until 15 months. The reproductive life of the average female is short. (Redford & Eisenberg 1992, Castro-Arellano et al. 2000). This species is considered an "r-strategist", in other words its reproductive behaviour favours a rapid rate of population increase. Such strategies are typical of species inhabiting short-lived environments or those that undergo large fluctuations in population size (Hershkovitz 1997).
BEH: General Behaviour Adults are generally solitary and as with many opossums they are not territorial and occupy home ranges that may broadly overlap. There is little or no contact or antagonism between adults provided that sufficient resources exist (Redford & Eisenberg 1992) and no dominance hierarchy amongst adults. Several animals fed nightly at an artificial feeding table in French Guiana, antagonism being generally exhibited only during the first hours of the night when animals arrived for their first feed - typically the arriving (ie more hungry) animal would chase away the animal that was already feeding. Only on three occasions was actual physical violence witnessed, taking the forming of biting, but threat displays were frequent (Charles-Dominique 1983). Home range size is variable depending on the availability of resources and the animals are quite nomadic (Castro-Arellano et al. 2000). Activity is generally nocturnal and the species is largely terrestrial when foraging though somewhat semi-arboreal in overall behaviour (Massoia et al 2002, Nowak 1991). On release from a trap 93% of animals in Brazil and 94% of animals in Peru used terrestrial escape routes (Castro-Arellano et al. 2000). In Surinam the species is reported to be just as active by day as it is by night (Husson 1978). Movements are rapid and agile and all 46 specimens captured by Handley (1976) in Venezuela were taken on the ground in wet areas. They swim well (Emmons 1999) and run with a trotting gait, using leaps and bounds only when trying to make a rapid escape (Castro-Arellano et al. 2000). Though they are not highly arboreal, they climb well, using the prehensile tail as fifth limb used for swinging the body from limb to limb and the hands for grasping for higher support. They will also use the tip of the tail for clasping a high branch and then actively climb up the tail to reach it. Animals descending tree-trunks do so head first or by simply jumping to the ground (Hershkovitz 1997). Captive individuals live for 2 to 4 years (Nowak 1991), but based on dental wear wild individuals are unlikely to survive more than 2.5 years (Castro-Arellano et al. 2000). The eyes shine orange under torchlight (Hershkovitz 1997). Refuges Nests are globular with a diameter of c30cm, constructed largely of dry plant matter and may be built at a height of 8 to 10m above the forest floor. Typically they are situated in holes in trunks or forks of trees, or else closer to the ground, for example in holes in fallen trunks. Ground nests, frequently among tree roots and shallow burrows are also reportedly occupied (Nowak 1991) and there are records of nests located in the thatched roofs of abandoned buildings. Grey Four-eyed Opossums sleep curled into a ball, and though the true eyes are not visible, the "false eyes" are and give the appearance of an animal that is alert and awake (Castro-Arellano et al. 2000). Grooming The species grooms for longer periods than Didelphis opossums but in a similar manner, cleaning the muzzle with the hands in a mouse-like fashion, and the rest of the pelage with the tongue in a cat-like manner. A captive female kept by Hershkovitz (1997) frequently washed the face after and during meals, using one or both paws, usually following by grooming the forequarters, sides and rump, but paying little or no attention to her pouched young. A captive pair in Colombia invariably defecated into their water bowl rather than elsewhere (Tyndale-Briscoe 1980). Defensive Behaviour Disturbed Philander will generally climb trees to avoid danger, but when cornered this species threatens with the mouth open whilst making hissing sounds (Emmons 1999, Massoia et al 2002) and is prepared to defend itself aggressively (Nowak 1991). Animlas have been recorded to dive into water to escape pursuers and swim away beneath the surface. Herskovitz (1997) notes that cornered animals may adopt a bipodal or tripodal stance and lurch forward at the agressor with open mouth. Considered the most aggressive opossum species after Metachirus by Enders (1935), who noted that an individual shipped live with a Metachirus of similar size had been "thoroughly cowed" by it and showed signs of "being bitten severely about the head and neck". Animals caught in steel traps bite violently at the bars, even if they break their teeth. They are not known to feign death. Hershkovitz (1997) documents a bizarre case of spontaneous bleeding in a captive animal in Colombia. The animal would bleed from the tips of the fingers, tail and nose when irritated. The animal had been clubbed by its captor, but had recovered without any apparent ill effects, but it is not known whether such bleeding was associated with its mode of capture. Stressed animals coil the tail under the body. Enemies Castro-Arellano et al. (2000) listed Ocelot, Jaguarundi, Tayra as potential enemies as well as large owls such as Barn Owl. Wilson (1970) described an aggressive encounter with a Didelphis marsupialis in Panama in which the two animals circled each other and attempted to bite each others rostrum, with the Didelphis eventually subduing and killing the Philander and consuming it. Parasites Numerous parasites have been documented in this species across its wide range. For example the parasitic fungus Histoplasma capsulatum; Protozoans (Babesia brasiliensis, Besnoitia sp., Haemogregorina sp., Sarcocystis garnhami, Tyrpanosoma cruzi); Nematode worms (Aspidodera sp., Capillaria sp., Cortiamosoides sp., Cruzia tentaculata, Globocephalus marsupialis, Gnatosthoma sp., Gongylonemoides sp., Macielia sp., Oxysoma sp., Philostrongylus sp., Physaloptera sp., Skrjabinofilaria sp., Subulura sp., Travassostrongylus sp., Trichuris sp., Viannaia barusi, V.conspicua, V.minispicula, V.skrjabini, V.tenorai, V.vianniai); Trematodes (Amphimeruse ruparupu, Brachylaemus sp., Duboisiella proloba, Maritrema sp., Opistorchis sp., Paragonimus amazonicus, Phaneropsolus sp., Plagiorchis didelphidis, Platynosomum sp., Podospathalium sp, Zonorchis allentoshi); Cestode worms (Linnistowia iheringi, Oochoristica brasiliensis, Sparganum reptans); Malliphagian lice (Gliricolla porcelli, Gryopus ovalis, Trimenophon hispidus); Fleas (Adoratopsylla intermedia, A.antiquorum, Ctenocephaloides felis, Neotyplocercus rosenbergi, Polygenis roberti, P.klagesi, Pulex sp., Rhopalopsyllus australis, R.cacius, R.lutzi, Tritopsylla intermedia, Xenopsylla cheopsis); Mites, ticks and other Acari (Amblyomma auricularium, A.geayi, Androlaelaps fahrenholzi, Archemyobia pectinata, Crotiscus disdentatus, Euschoengastia nunezi, Eutrombicula alfreddugesi, E.goeldii, E.tropita, Gigantolaelaps sp, Haemolaelaps glasgowi, Heterothrombidium sp., Intercutestrix sp., Ixodes lasallei, I.luciae, I.venezuelensis, Leeuwenhoekia sp., Microthrombidium sp., Neotyphloceras sp., Ornithonyssus wernecki, Pentastoma sp., Porocephalus sp., Pseudoschoengastia bulbifera, Shongastia sp., Trimenopon sp., Trombicula dunni, T.keenani, Tur apicalis, T.uniscutatus). (Castro-Arellano et al. 2000).
VOC: Hissing noises accompany threat behaviour and they may utter a "long, chattering cry" when disturbed (Massoia et al 2002, Nowak 1991). Clicks, chirps and hisses are used in communication (Redford & Eisenberg 1992). In the presence of danger females call their young with a dry tik tik tik call which encourgaes the young to immediately run to her for her refuge. Juveniles begin to give clicking calls about one month before detatching from the nipple and soon begin to give the hissing threat call. Sexual calls by adults are quiet, mouse-like squeaks. (Charles-Dominique 1983).
HUM: Occasional damage to fruit crops and cornfields has led to them receiving a bad reputation in parts of their range where they are more abundant (Cimardi 1996). In Paraguay this species is rarely encountered and interviews with locals performed by Lowen et al (1996) failed to find any reference to the species. Its human impact in Paraguay is therefore minimal. The species acts as a reservoir for Trypanosoma cruzi and is eaten in certain parts of its range eg Guyana, though the flesh is said to be foul-smelling. It does not figure as a regular dietary item for the indigenous tribes in Paraguay, but with infection rates varying between 5% and 40% in French Guiana it is a potential source of infection.
CON: Globally considered to be of Low Risk Least Concern by the IUCN, click here to see their latest assessment of the species. It is likely under-recorded in Paraguay but has no doubt disappeared from large areas of its former range as a result of conversion of forest to agriculture. Population density in Panama was estimated at 0.55 to 0.65ha (Nowak 1991) with the highest densities in the dry season and in Mexico at 0.48/ha with an average biomass of 176g/ha. Both sexes moved an average of 47.1m between captures with the greatest movement 117m by a male. At a secondary forest site in French Guiana an average of 137/km2 was estimated with a biomass of 55.8kg/km2. This population was highly mobile and 97% of the adult population changed with less than 17% of the population remaining in the study area for more than 200 days. At a primary forest site in the same country the density was lower with an estimate of just 17/km2 and a biomass of 7kg/km2. (Castro-Arellano et al. 2000).
Citable Reference: Smith P (2007) FAUNA Paraguay Online Handbook of Paraguayan Fauna Mammal Species Account 9 Philander opossum.
Last Updated: 4 August 2008.
References:
Allen JA 1900 - Note on the Generic Names Didelphis and Philander - Bulletin AMNH 13: p185-190.
Brown BE 2004 - Atlas of New World Marsupials - Fieldiana Zoology 102.
Castro-Arellano I, Zarza H, Medellín RA 2000 - Philander opossum - Mammalian Species 638: p1-8.
Charles-Dominique P 1983 - Ecology and Social Adaptations in Didelphid Marsupials: Comparison with Eutherians of Similar Ecology p395-422 in Eisenberg JF, Kleiman G eds Advances in the Study of Mammalian Behavior - American Society of Mammalogists Special Publication 40.7.
Charles-Dominique P, Atramientowicz M, Charles-Dominique M, Gerard H, Hladik A, Hladik CM, Prevost MF 1981 - Les Mammifères Frugivorés Arboricolés Nocturnés d´une Forete Guyanaise - Revue Ecologie 35: p342-435.
Cimardi AV 1996 - Mamíferos de Santa Catarina - FATMA, Florianopolis.
Cuéllar E, Noss A 2003 - Mamíferos del Chaco y de la Chiquitania de Santa Cruz, Bolivia - Editorial FAN, Santa Cruz.
Eisenberg JF 1989 - Mammals of the Neotropics: Volume 1 The Northern Neotropics - University of Chicago Press, Chicago.
Eisenberg JF, Redford KH 1999 - Mammals of the Neotropics: Volume 3 The Central Neotropics - University of Chicago Press, Chicago.
Emmons LH 1999 - Mamíferos de los Bosques Húmedos de América Tropical - Editorial FAN, Santa Cruz.
Enders RK 1935 - Mammalian Life Histories from Barro Colorado Island, Panama - Bulletin of the Museum of Comaerative Zoology 78: p385-502.
Esquivel E 2001 - Mamíferos de la Reserva Natural del Bosque Mbaracayú, Paraguay - Fundación Moises Bertoni, Asunción.
Fleming TH 1973 - The Reproductive Cycles of Three Species of Opossums and Other Mammals in the Panama Canal Zone - Journal of Mammalogy 53: p439-455.
Fonseca SD, Cerqueira R 1991 - Water and Salt Balance in a South American Marsupial, the Gray Four-eyed Opossum Philander opossum - Mammalia 55: p421-432.
Handley CO 1976 - Mammals of the Smithsonian Venezuelan Project - Brigham Young Scientific Bulletin Biological Series 20 (5): p1-89.
Hershkovitz P 1997 - Composition of the Family Didelphidae Gray 1821 (Didelphoidea: Marsupialia) with a Review of the Morphology and Behaviour of the Included Four-eyed Pouched Opossums of the Genus Philander Tiedemann 1808 - Fieldiana 86.
Husson AM 1978 - The Mammals of Surinam - EJ Brill, Leiden.
Lew D, Pérez-Hernandez R, Ventura J 2006 - Two New Species of Philander (Didelphimorphia: Didelphidae) from Northern South America - Journal of Mammalogy 87: p224-237.
Lowen JC, Bartrina L, Clay RP, Tobias JA 1996 - Biological Surveys and Conservation Priorities in Eastern Paraguay - CSB Conservation Publications, Cambridge.
Massoia E, Forasiepi A, Teta P 2000 - Los Marsupiales de la Argentina - LOLA, Buenos Aires.
Myers P, Espinosa R, Parr CS, Jones T, Hammond GS, Dewey A 2006 - The Animal Diversity Web (online). Accessed December 2007.
Novak RM 1991 - Walker´s Mammals of the World 5th Ed Volume 1 - Johns Hopkins, Baltimore.
Patton JL, da Silva MFN 1997 - Definition of Species of Pouched Four-eyed Opossums (Didelphidae: Philander) - Journal of Mammalogy 78: p90-102.
Pine RH 1973 - Anatomical and Nomenclatural Notes on Opossums - Proceedings of the Biological Society of Washington 86: p391-402.
Redford KH, Eisenberg JF 1992 - Mammals of the Neotropics: Volume 2 The Southern Cone - University of Chicago Press, Chicago.
Tyndale-Biscoe CH 1970 - Observations on the Biology of Marsupials in Colombia and Venezuela - Research Reports of the National Geographic Society 12: p711-720.
Wilson DE 1970 - Opossum Predation: Didelphis on Philander - Journal of Mammalogy 51: p386-387.


