

Designed by Paul Smith 2006. This website is copyrighted by law.
Material contained herewith may not be used without the prior written permission of FAUNA Paraguay.
Photographs on this web-site and are used with permission.
Micoureus paraguayanus (Tate 1931) Image Gallery
TAX: Class Mammalia; Subclass Theria; Infraclass Metatheria; Order Didelphimorphia; Family Didelphidae; Subfamily Didelphinae, Tribe Monodelphini (Myers et al 2006, Gardner 2007). The genus Micoureus was defined by Lesson, 1842. There are six known species according to the latest revision (Gardner 2007) only one of which is present in Paraguay. The genus Micoureus is probably taken from the Guaraní/Tupi indigenous name for an opossum Mykuré. The species name paraguayanus refers to Paraguay the country of provenance of the type specimen. The species is monotypic. Formerly considered conspecific with the Woolly Mouse Opossum M.demerarae (O.Thomas, 1905), the Atlantic Forest form was described by Tate (1931) as Micoureus cinerea paraguayanus, with type locality "Villarica, Paraguay". Tate (1933) was unwittingly referring to this species in part when making his description of Micoureus cinerea, though for the cranial characteristics he relied on three different specimens from Pará. Pernambuco and Rio de Janeiro. As currently understood only the Rio de Janeiro specimen would today be referrable to M.paraguayanus, the others likely being M.demerarae dominus (Gardner 2007). More recently the names Micoureus limae (Patton, Silva & Malcolm 2000) and Micoureus travassosi (Patton & Costa 2003) have been used for the species. Synonyms adapted from Gardner (2007):
Didelphis cinerea Temminck 1824:46. Type locality "Brésil" restricted by Tate 1933:55 to Río Mucurí, Bahía, Brazil.
Micoureus cinereus Lesson 1842:186. Name combination.
Philander cinerea Gray 1843:101. Name combination.
Didelphys [(Metachirus)] cinerea Burmeister 1854:137. Name combination.
Didelphys [(Micoureus)] cinerea O.Thomas 1888:342. Name combination.
Grymaeomys cinerea Winge 1893:46. Name combination and incorrect gender.
[Didelphys (Marmosa)] cinerea Trouessart 1898:1238. Name correction.
Marmosa cinerea O.Thomas 1901:536. Name combination.
[Didelphis (Caluromys)] cinerea Matschie 1916:269. Name combination.
[Marmosa (Marmosa)] cinerea cinerea Cabrera 1919:36. Name combination.
Marmosa (Micoureus) cinerea Pohle 1927:241. Name combination.
Marmosa cinerea paraguayana Tate 1931:1. Type locality "Villa Rica", Guairá, Paraguay.
[Micoures] cinereus Reig, Kirsch & Marshall 1985:342. Name combination.
Micoures cinerea paraguayana Massoia 1988:6. Name combination and incorrect gender.
[Micoureus demerarae] paraguayana Gardner 1993:20. Name combination and incorrect gender.
Micoureus cinereus paraguayanus González, Marques & Pacheco 1997: 195. Name combination.
[Micoureus] limae Patton, Silva & Malcolm 2000:72. Name combination.
[Micoureus] travassosi Patton & Costa 2003:75. Name combination.
Micoureus paraguayensis Gardner 2007:77. Name combination and transcription error.
ENG: Long-furred Woolly Mouse Opossum (Brito & Fonseca 2007, Canevari & Vaccaro 2007), Tate´s Woolly Mouse Opossum (Gardner 2007), Woolly Mouse Opossum (Barros et al 2008).
ESP: Marmosa grande gris (Redford & Eisenberg 1992, Massoia et al 2000), Comadrejita cenicienta (Massoia et al 2000), Comadrejita gris (Canevari & Vaccaro 2007), Marmosa lanuda de pelo largo (Emmons 1999).
GUA: Anguyá-mykuré (Massoia et al 2000), Guaikí (Massoia et al 2000).
DES: A large stocky mouse opossum with relatively short snout and long, thick, woolly pelage. Head somewhat triangular in profile. Dorsal pelage uniform greyish, sometimes with a slight brownish tinge. Ventrally creamy-yellow or buffy-white, the colour extending onto the chin, laterally towards the cheeks and on the face up between the eyes. Eyes large and dark, accentuated by black patches around the eyes which extend slightly in a point towards the snout. Ears large, slightly pointed but with rounded tips and brownish-pink in colour. Nose pinkish. Feet are broad and pinkish, the claws of the forefeet extending slightly beyond the digital pads. Thenar and first interdigital pads are fused on the hindfoot but lie together on the forefoot. Fourth interdigital pad lies against the hypothenar pad of the forefoot but the two are either fused or in direct contact on the hindfoot. Central part of the soles of all feet are smooth. Digit IV on the hindfoot is longest with a length ratio of 0.45 when compared to the hindfoot length. Second and third interdigital pads on all feet are triangular and approximately as wide as they are long. Ventral surfaces of the digits have transverse bars. Tail long (c1.3x head and body length) and furred for 3-5cm at the base. Tail with sparse hair, characteristically bicoloured with a blackish-brown base and pinkish white terminal third. Tail scales are rhomboid and arranged in a spiral. Females lack a marsupium but have 11 inguinal and abdominal mammae arranged in a circular pattern (5-1-5). Male with bluish scrotum. (Tate 1933, Emmons 1999, Massoia et al 2000, Canevari & Vaccaro 2007, Gardner 2007). CR - Zygomata evenly arched and broadly expanded, but converging anteriorly so that the greatest width of zygomatic arch is near the junction of the squamosals. Supraorbital processes slightly pointed and located anteriorly when compared to other members of the genus. Nasals broad basally. Temporal ridges not closely approximated and postorbital constriction is not marked. Bullae large and well-rounded. Palate short and broad.(Tate 1933). DF: I5/4 C1/1 P 3/3 M 4/4 = 50. I1 is longest and separated from I2 by a space. Incisor length increases from I2 through to I5. P2 is larger than P3 and M3 is the widest upper molar. Canines are long and curved. CN: 2n=22.
TRA: No information.
MMT: Easily the largest of the Paraguayan Mouse Opossums. TL: 38.88cm (27-46cm); HB: 16.86cm (12-20cm); TA: 21.94cm (15-26cm); FT: 2.51cm (2.25-2.95cm); EA: 2.75cm (2.5-3cm); WT: male 109.9g (56-194g) female 99.1g (53-230g). (Redford & Eisenberg 1992). The following mean post-cranial measurements were noted by Carvalho et al (2000) for Brazilian specimens (n=5): Ulna 29mm; Forearm 31.4mm; Humerus 25.8mm; Tibia 30.2mm; Foreleg 37.1mm; Femur 31mm.
SSP: Identifiable by size alone, this is much the largest of the Paraguayan mouse opossums. Note also the dense, woolly, greyish pelage and the bicoloured tail with dark base and whitish tip, which immediately identifies the species in Paraguay. Both Gracilinanus agilis and Cryptonanus chacoensis are considerably smaller (with body length approximately the length of an index finger as opposed to an entire hand in this species). Thylamys macrurus is the only species that approaches this in size but it lacks the bicoloured tail (though it is white-tipped) and has notably constrasting pelage with the flanks paler than the dorsum. Furthermore female Thylamys have the teats arranged in bilaterally symmetrical rows and not in a circular pattern as in other mouse opossums. Finally note that this species is confined to Atlantic Forest habitat and would not be expected in the Chaco or dry areas. A second species of Micoureus, M.constantiae, occurs in the cerrado and Chaco of Paraguay. Though of similar general shape and sharing the woolly pelage of M.paraguayanus (though less dense), the species can be distinguished on account of its more reddish dorsal pelage and the fact that the fur does not extend notably over the base of the tail as it does in paraguayanus. Crucially the ventral pelage is basally whitish in constantiae and grey-based in paraguayanus. Note also that constantiae looks much yellower and paler on the facial area, especially on the snout and around the black eye-rings.
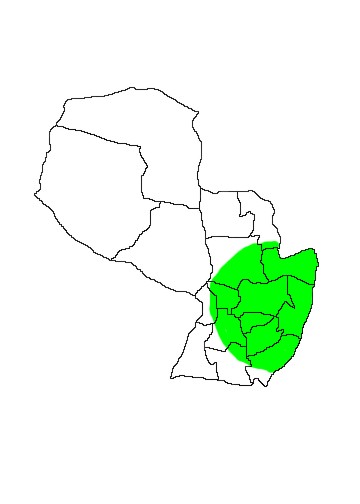
DIS: This species is restricted to eastern Paraguay, Provincia Misiones in Argentina (Iguazú, Gral. Belgrano, Candelaria, Cainguás, Oberá, San Ignacio and Apóstoles - Chebez 1996) and eastern Brazil from southern Bahía to Rio Grande do Sul. In Paraguay specimens are known from Departamentos Paraguarí (Sapucai), San Pedro (Nueva Germania), Guairá (Villarica), Itapúa (PN San Rafael) and Alto Paraná (Itabó Itaipú Reserve and Tati Yupi). It was not listed by Esquivel (2001) for the Mbaracayú Biosphere Reserve, Departamento Canindeyú, but it is likely present there.
HAB: This species is considered endemic to the Atlantic Forest (Barros et al 2008) with a preference for areas of dense forest rich in vines and palm trees, though it also occurs in open, high forest (Emmons 1999). In Brazil it is able to tolerate a certain degree of habitat disturbance and fragmentation and occurs in both secondary and primary forest, having even been recorded in exotic Eucalyptus plantations with a native subcanopy (Stallings 1989). Pires et al (2002) found that only 1.2% of 442 recaptures showed evidence of movement between forest fragments in Rio de Janiero State, Brazil.
ALI: Carvalho et al (1999) found that in Rio de Janeiro State, Brazil the most frequent items in fecal samples (n=105) were insects from the orders Coleoptera (in 63.3% of fecal samples), Hymenoptera (56%), Arachnida (25.7%), Orthoptera (19.3%), Hemiptera (15.6%), Lepidoptera pupae and larvae (14.7%), Diptera pupae and larvae (9.2%) and Blattaria (1.8%). Smaller amounts of Neuroptera and termites (0.9% each) were also recorded. Surprisingly freshwater Crustacea (Copepoda and Isopoda) were noted in 0.9% of the fecal samples. 64% of fecal samples contained seeds mainly from secondary vegetation, those that were identified including Piper (23.5%), Cecropia (10.9%) and Solanaceae (1.6%). Casella & Cáceres (2006) investigated stomach contents of the species in Paraná State, Brazil (n=3) and considered the species to be an opportunistic generalist feeder with the emphasis on insectivory supplemented by frugivory and carnivory in smaller quantities. They found Blattaria, Hymenoptera and bird remains in two out of the three specimens, and Orthoptera in one specimen. Seeds of Cecropia sp. and an unidentified species of Solanaceae were found in single different specimens. During a fecal analysis study (n=30) on Santa Catarina Island, Brazil, Cáceres et al (2002) found Coleoptera (53% of samples) and Hymenoptera (43%) to be the main items in the diet. Decapoda were present in 33% of sample and Opiliones in 23%. Other animal items present in the diet were: Birds (7%), Blattaria (7%), Lepidoptera (7%), Diplopoda (7%) and Orthoptera (3%). Seeds of Cecropia (33%), Piper sp (27%) and Ottonia martiana (23%) were prominent in the diet, andin lesser quantities Ficus sp. (7%) and Maclura tinctoria (7%). Larger quantities of seeds (e.g. Cecropia, Piper, Ficus and Ottonia) appeared in feces during the warmer and rainy months (March to May) and became absent or less prominent during the colder months (June to August). They also noticed a correlation in that fruit was more prominent in the diet when the species was trapped in the trees and less prominent when trapped on the ground, suggesting that an absence or reduction in arboreal fruit is related to descent to the ground to feed. The presence of intact seeds in the diet likely represents frugivory rather than granivory and the species is presumably an important seed disperser in forest fragments. Pinheiro et al (2002) studied diet through fecal samples at Poço das Antas. They found that diet composition was constant between sexes, seasons, age classes and changing climactic conditions. They found a slightly greater diversity of arthropods in the non-breeding versus the breeding season but considered it to be an artefact of sampling. They concluded that the species is an opportunistic feeder. The following arthropods were recorded, with the number representing the percentage of the total samples (n=98) in which they were found: Coleoptera (59%), Hymenoptera (55%), Arachnida (24%), Orthoptera (21%), Hemiptera (16%), Lepidopteran larvae (15%), Diptera larvae (8%), Blattaria (2%), Neuroptera (2%), Corrodentia (1%), Copepoda (1%), Isopoda (1%). The crustacean orders (aquatic Copepoda and terrestrial Isopoda) represented the first recording of this invertebrate group in the diet of this species. Seeds in the feces mostly belonged to secondary species (Cecropia sp 13%, Ficus sp 2% and Piper sp 27%) and were indicative of opportunistic frugivory. In fact the amount of fruit consumed is likely to be underestimated by fecal analysis given that fruit is more easily digested than animal matter and passes through the digestive system more rapidly. Cáceres et al (2002) captured individuals in traps baited with banana and peanut butter. Astúa de Morães et al. (2003) experimentally tested the proportions of protein, lipid, carbohydrate and fibre in the diet of adults (n=7) of this species under laboratory condtions. Mean proportions per 100g dry weight of food were: protein 2.30g (+/-1.59); lipid 0.63g (+/-0.94); carbohydrate 8.13g (+/-1.53); fibre 2.81% (+/-0.44). Santori et al (2004) described and illustrated the gut morphology of this species and associated it with dietary habits.
REP: Barros et al (2008) documented the reproductive pattern of the species in Rio de Janeiro State, Brazil. The species was found to be strongly seasonal in its reproduction, corresponding with the time of year when resources are most plentiful. Females were found to be reproductively active only during the wet season (October-May) and juveniles were found only from January to May. At least two litters were produced annually, one in October/November and another in January/February. Litter size varied from 6 to 11. Pires & Fernández (1999) found territorial behaviour in this species to be consistent with a promiscuous mating system. The age of first breeding for both sexes is 6 months (Rocha 2000).
BEH: Activity Levels Arboreal and nocturnal, occupying the canopy and subcanopy of forest and only rarely descending to the ground. Locomotion Delciellos & Vieira (2006) studied arboreal locomotion of this species on horizontal branches in PN Serra dos Orgãos, Rio de Janeiro State, Brazil. A maximum velocity of 6.40 (+/-0.12) x body length/second was recorded on support branches of 10.12cm diameter, and a minimum velocity of 5.20 (+/-0.11) x body length/second was recorded on support branches of 2.54cm diameter. Minimum number of strides per second was 4.60 (+/-0.09) on support branches of 2.54cm and maximum number of stride lengths per second was 5.67 (+/-0.17) on support branches of 10.12cm diameter. Range of stride length was from 1.10 to 1.13 x body length. Maximum velocity is reached by increasing stride frequency (Delciellos & Vieira 2007). Delciellos & Vieira (2009) investigated climbing performance of this species on nylon ropes of three diameters 0.6cm, 0.9 and 1.25cm. Respective velocities (stride length x stride frequency) of 1.68 (+/-0.92), 2.01 (+/-0.95) and 2.48 (+/-0.85) were recorded for the three rope diameters. Number of strides per second respectively were 2.69 (+/-0.89), 3.26 (+/-1.80) and 2.99 (+/-0.74) for the three rope diameters. Stride length when related to body length was 0.60 (+/-0.18), 0.64 (+/-0.15) and 0.82 (+/-0.12) respectively. Home Range Pires & Fernández (1999) studied home range of the species in forest fragments in Rio de Janiero state, Brazil using a capture recapture technique. They found no significant difference in size between male and female home range, but male ranges increased in size during the breeding season. Over the course of the year mean home range size in males (n=10) was 0.82ha (range 0.1-2.45ha), increasing from a mean of 0.25ha (range 0.1-0.4ha) in the non-breeding season to 1.62ha (range 0.65-2.45ha) in the breeding season. Male ranges overlapped greatly during the breeding season, but during the non-breeding season they did not overlap with those of other males, though they did overlap with females. Female range size (n=16) remained constant throughout the year with a mean of 0.45ha (range 0.1-1.1ha) and there was little or no overlap in territorial boundaries. Range size of females was smaller where population density was greater. Dispersal between forest fragments was recorded in males only and only during the breeding season, the species forming a metapopulation of resident populations connected by a small number of individuals that move between populations (Brito & Fernández 2000, 2002). Moraes & Chiarello (2005) working in the same area used radiotracking to estimate home ranges and found larger home ranges for the species than the estimates obtained using capture recapture analysis. They estimated male home range to be 5.4-24ha and female home range at 0.3-10.7ha. Tagged individuals moved a mean of 423m per night (range 34-1140m), with males (mean 583m +/-53m; range 317-1097m) moving significantly further than females(mean 335m +/-47m; range 34-1014m). Areas of intense activity were associated with the nesting site and typically males had several areas of intense activity within their ranges whilst females had only one. Roosts Roosting behaviour of the species in Rio de Janeiro State, Brazil was studied by Moraes & Chiarello (2005). Roosts were occuppied during the daylight hours and the same roost site may be used more than once. A distinct preference for roosting in the spiny palm species Astrocaryum aculeatissimum was noted with 70.7% of the 58 roost sites found being in the junction between the petiole and trunk at a height of 4.55m (+/-1.36). Such sites were found to naturally gather masses of dry leaves which acted as a ready-made nest. Other roosts were in a tangle of lianas (n=7) and tree holes (n=2) at a mean height of 10.67m (+/-2.75m). The height difference was significant and it was hypothesised that the spiny trunk of the palm provided protection against predators and made it a favoured roost site. Defensive Behaviour Threatened animals gesture with the mouth open bearing the teeth, but the bite is weak. Enemies The species was predated by Barn Owl Tyto alba in Misiones Argentina (Massoia 1988). Parasites Limardi (2006) notes the following ectoparasites from Brazilian specimens: Siphanoptera Adoratopsylla ronnai (Ctenophthalmidae). Acari: Mesostigmata Bdellonyssus sp. (Macronyssidae). Acari: Astigmata Didelphoecius didelphicola (Atopomelidae). Longevity The longest lifespan recorded for a wild individual is 24 months (Rocha 2000).
VOC: No information.
HUM: None.
CON: Globally considered to be of Low Risk Least Concern by the IUCN, click here to see their latest assessment of the species on account of its wide distribution and presence in a number of protected areas. The species is able to tolerate some degree of habitat modification but its reliance on the endangered Atlantic Forest habitat means that it has undoubtedly declined substantially in recent years. Moderate habitat fragmentation probably has little affect on this species given its small size and they have been shown to cross 800m of open habitat between forest patches (Pires et al 2005). It has been suggested that the species may even prefer secondary forest (Pires et al 2005), though it is generally considered to be more numerous in pristine forest (Emmons 1999). Pires et al (2005) found that the species was captured less often near forest edge after fire than before fire, meaning that the combined effects of fire and fragmentation would likely act to reduce populations. Furthermore it has been suggested that only males disperse (Pires & Fernández 1999) and males alone cannot colonise empty patches of forest that are not already inhabited by females. However Moraes & Chiarello (2005) called this assumption into question following their radiotracking survey which apparently indicated that movements assumed to be dispersal may actually be part of normal but rare foraging patterns taking the animals into open habitats. Population sizes are typically small with estimates of less than 20 individuals in two Brazilian forest fragments of 7 and 8.8ha respectively (Quental et al 2001). Using a computer analysis of minimum viable population size, Brito & da Fonseca (2006) estimated that populations of 100 and 2000 individuals were necessary to achieve demographic and genetic stability respectively, within a time frame of 100 years and that isolation of populations represented the greatest threat to their survival. Minimum area of suitable habitat was estimated as 65ha to preserve demographic stability and 1300ha to preserve genetic stability. Given the fact that resources are not evenly distributed within any one area of forest the conservation of larger areas is required to effectively conserve the species. Brito & Grelle (2004) had earlier estimated that a minimum reserve size of 3600ha was necessary to maintain a viable population in Rio de Janiero State, Brazil. Brito & da Fonseca (2007) ran a computer simulation to model the effects of population fragmentation and found that a single population was more stable than several smaller populations of equal size regardless of the rate of dispersal. Furthermore they concluded that populations of <50 individuals were highly susceptible to extinction over a 100 year time frame. Working under the assumption of male-biased dispersal, Brito & da Fonseca (2006) recommended promoting conditions for dispersal jointly with translocation of females as the best means of conserving the species. Given the increasingly fragmented nature of the Atlantic Forest in Paraguay, the species might best be considered near threatened nationally.
CITE AS: Smith P (2009) FAUNA Paraguay Online Handbook of Paraguayan Fauna Mammal Species Account 30 Micoureus paraguayanus.
LAST UPDATED: 30 June 2009.
REFERENCES:
Astúa de Morães D, Santori RT, Finotti R, Cerquiera R 2003 - Nutritional and Fibre Contents of Laboratory-established Diets of Neotropical Opossums (Didelphidae) p225-233 in Jones M, Dickman C, Archer M Predators with Pouches: The Biology of Carnivorous Marsupials - CSIRO Publishing, Australia.
Barros CS, Crouzeilles R, Fernández FAS 2008 - Reproduction of the Opossums Micoureus paraguayanus and Philander frenata in a Fragmented Atlantic Forest Landscape in Brazil: Is Seasonal Reproduction a General Rule for Neotropical Marsupials? - Mammalian Biology 73: p463-467.
Brito D, da Fonseca GAB 2006 - Evaluation of Minimum Viable Population Size and Conservation Status of the Long-furred Woolly Mouse Opossum Micoureus paraguayanus: An Endemic Marsupial of the Atlantic Forest - Biodiversity and Conservation 15: p1713-1728.
Brito D, da Fonseca GAB 2007 - Demographic Consequences of Population Subdivision on the Long-furred Woolly Mouse Opossum Micoureus paraguayanus from the Atlantic Forest - Acta Oecologia 31: p60-68.
Brito D, Fernández FAS 2000 - Metapopulation Viability of the Marsupial Micoureus demerarae in Small Atlantic Forest Fragments in Southeastern Brazil - Animal Conservation 3: p201-209.
Brito D, Fernández FAS 2002 - Patch Relative Importance to Metapopulation Viability: The Neotropical Marsupial Micoureus demerarae as a Case Study - Animal Conservation 5: p45-51.
Brito D, de Viveiros Grelle CE 2004 - Effectiveness of the Reserve Network for the Conservation of the Endemic Marsupial Micoureus travassosi in Atlantic Forest Remnants in Southeastern Brazil - Biodiversity and Conservation 13: p2519-2536.
Burmeister H 1854 Systematische Uebersicht der Thiere Brasiliens: Welche während einer Reise durch die Provinzen von Rio de Janeiro und Minas Geraës Gesammlt oder beobachtet wurden Vol 1 - G.Reimer, Berlin.
Cabrera A 1919 - Genera Mammalium. Monotremata, Marsupialia - Museo Nacional de Ciencias Naturales, Madrid.
Cáceres NC, Ghizoni IR, Graipel ME 2002 - Diet of Two Marsupials Lutreolina crassicaudata and Micoureus demerarae in a Coastal Atlantic Forest Island in Brazil - Mammalia 66: p331-340.
Cannevari M, Vaccaro O 2007 - Guía de Mamíferos del Sur de América del Sur - LOLA, Buenos Aires.
Carvalho FMV de, Delciellos AC, Vieira MV 2000 - Medidas Externas dos Miembros de Marsupiais Didelfidios: Uma Comparação com Medidas do Esqueleto Pós-Craniano - Boletim do Museu Nacional, Rio de Janeiro 438.
Carvalho FMV de, Pinheiro PS, Fernández FAS, Nessimian JL 1999 - Diet of Small Mammals in Atlantic Forest in Southeastern Brazil - Revista Brasileira de Zoociencias 1: p91-101.
Casella J, Cáceres NC 2006 - Diet of Four Small Mammal Species from Atlantic Forest Patches in South Brazil - Neotropical Biology and Conservation 1: p5-11.
Chebez JC 1996 - Fauna Misionera - LOLA, Buenos Aires.
Delciellos AC, Vieira MV 2006 - Arboreal Walking Performance in Seven Didelphid Marsupials as an Aspect of Their Fundamental Niche - Austral Ecology 31: p449-457.
Delciellos AC, Vieira MV 2007 - Stride Lengths and Frequencies of Arboreal Walking in Seven Species of Didelphid Marsupials - Acta Theriologica 52: p101-111.
Delciellos AC, Vieira MV 2009 - Allometric, Phylogenetic and Adaptive Components of Climbing Performance in Seven Species of Didelphid Marsupials - Journal of Mammalogy 90: p104-113.
Eisenberg JF, Redford KH 1999 - Mammals of the Neotropics: Volume 3 The Central Neotropics - University of Chicago Press, Chicago.
Emmons LH 1999 - Mamíferos de los Bosques Húmedos de América Tropical - Editorial FAN, Santa Cruz.
Esquivel E 2001 - Mamíferos de la Reserva Natural del Bosque Mbaracayú, Paraguay - Fundación Moises Bertoni, Asunción.
Gardner AL 1993 - Order Didelphimorphia p15-24 in Wilson DE, Reeder DM Mammal Species of the World - Smithsonian, Washington DC.
Gardner AL 2007 - Mammals of South America Volume 1: Marsupials, Xenarthrans, Shrews and Bats - University of Chicago Press.
González JC, Marques RV, Pacheco SM 1997 - Ocurrência de Micoureus cinereus paraguayanus (Tate) (Mammalia: Didelphidia: Marmosidae) no Rio Grande do Sul, Brasil - Revista Brasileira de Zoologia 14: p195-200.
Gray JE 1843 - List of the Specimens of Mammalia in the Collection of the British Museum - British Museum of Natural History, London.
Lesson RP 1842 - Nouveau Tableau do Règne Animal: Mamifères - Arthus-Bertrand, Paris.
Limardi PM 2006 - Os Ectoparasitos de Marsupiais Brasileiros p27-52 in Cáceres NC, Monteiro-Filho ELA Os Marsupiais do Brasil:Biologia, Ecologia e Evolução - Editora UFMS, Campo Grande.
Massoia E 1988 - Presas de Tyto alba en Campo Ramón, Departamento Obrera, Provincia de Misiones - Aprona Boletín Cientifico 7: p4-15.
Massoia E, Forasiepi A, Teta P 2000 - Los Marsupiales de la Argentina - LOLA, Buenos Aires.
Matschie P 1916 - Bemerkungen über die Gattung Didelphis L. - Sitzungsber. Gesells. Naturf. Freunde Berlin 1916: p259-272.
Moraes Jr EA, Chiarello AG 2005 - A Radiotracking Study of Home Range and Movements of the Marsupial Micoureus demerarae (Thomas) (Mammalia: Didelphidae) in the Atlantic Forest of Southeastern Brazil - Revista Brasileira de Zoologia 22: p85-91.
Moraes Jr EA, Chiarello AG 2005 - Sleeping Sites of Woolly Mouse Opossum Micoureus demerarae (Thomas) (Didelphimorphia: Didelphidae) in the Atlantic Forest of Southeastern Brazil - Revista Brasileira de Zoologia 22: p839-843.
Myers P, Espinosa R, Parr CS, Jones T, Hammond GS, Dewey A 2006 - The Animal Diversity Web (online). Accessed December 2007.
Novak RM 1991 - Walker´s Mammals of the World 5th Ed Volume 1 - Johns Hopkins, Baltimore.
Patton JL, Costa LP 2003 - Molecular Phylogeography and Species Limits in Rainforest Didelphid Marsupials of South America in Jones ME, Dickman FR, Archer M eds Predators with Pouches: The Biology of Carnivorous Marsupials - CSIRO, Collingwood, Australia.
Patton JL, da Silva MNF, Malcolm JR 2000 - Mammals of the Río Juruá and the Evolutionary and Ecological Diversification of Amazonia - Bulletin AMNH 244.
Pinheiro PS, Carvalho FMV, Fernandez FAS, Nessimian JL 2002 - Diet of the Marsupial Micoureus demerarae in Small Atlantic Forest Fragments in Southeastern Brazil - Studies on Neotropical Fauna and Environment 37: p213-218.
Pires AS, Fernandez FAS 1999 - Use of Space by the Marsupial Micoureus demerarae in Small Atlantic Forest Fragments in Southeastern Brazil - Tropical Ecology 15: p279-290.
Pires AS, Koeler Lira P, Fernandez FAS, de Freitas D 1999 - Patterns of Space Use by Micoureus demerarae (Marsupialia: Didelphidae) in a Fragment of Atlantic Forest in Brazil - Mastozoologia Neotropical 6: p39-45.
Pires AS, Koeler Lira P, Fernandez FAS, de Freitas D, Feliciano BR 2005 - Influence of Edge and Fire-induced Changes on Spatial Distribution of Small Mammals in Brazilian Atlantic Forest Fragments - Studies on Neotropical Fauna and Environment 40: p7-14.
Pires AS, Fernandez FAS, Schittini GM, Oliveira LC 2002 - Frequency of Movements of Small Mammals Along Coastal Atlantic Forest Fragments in Brazil - Biological Conservation 108: p229-237.
Pohle H 1927 - Über die von Prof. Bresslau in Brasilien Gesammelten Säugetiere (Auseen den Nagethieren) - Abhandl. Senckenberg. Naturf. Gesell. Frankfurt 40: p239-247.
Quental TB, Fernandez FAS, Dias ATC, Rocha FS 2001 - Population dynamics of the marsupial Micoureus demerarae in small fragments of Atlantic Coastal Forest in Brazil - Journal of Tropical Ecology 17: p339-352.
Redford KH, Eisenberg JF 1992 - Mammals of the Neotropics: Volume 2 The Southern Cone - University of Chicago Press, Chicago.
Reig OA, Kirsch JAW, Marshall LG 1985 - New Conclusions on the Relationships of the Opossum-like Marsupials with an Annotated Classification of the Didelphimorphia - Ameghiniana 21: p335-343.
Rocha FS 2000 - Ecologia reprodutiva de pequenos mamiferos (com enfase no marsupial Micoureus demerarae) em fragmentos de Mata Atlantica no sudeste do Brasil - MSc thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro.
Santori RT, Astúa de Moraes D, Cerqueira R 2004 - Comparative Gross Morphology of the Digestive Tract in Ten Didelphidae Marsupial Species - Mammalia 69: p27-36.
Stallings JR 1989 - Small Mammal Inventories in an Eastern Brazilian Park - Bulletin of Florida Museum of Natural History 4: p153-200.
Tate GHH 1931 - Brief Diagnoses of Twenty-six Apparently New Forms of Marmosa (Marsupialia) from South America - AMNH Novitates 493.
Tate GHH 1933 - A Systematic Revision of the Marsupial Genus Marmosa - Bulletin AMNH 66.
Temminck CJ 1824 - Deuxième Monographie sur le Genre Sarigue: Didelphis (Linn.) p21-52 in Monographies de Mammalogie ou Description de Quelques Genres de Mamifères dont les Espèces ont été Observeés dans les Différens Musées de l´Europe - G.Dufour & E d´Ocagne, Paris.
Thomas O 1888 - Catalogue of the Marsupialia and Monotremata in the Collection of the British Museum (Natural History) - British Museum of Natural History, London.
Thomas O 1901 - On Mammals Obtained by Mr Alphonse Robert on the Rio Jordão, SW Minas Geraes - Annals and Magazine of Natural History Series 7 8: p526-536.
Thomas O 1905 - New Neotropical Chrotopterus, Sciurus, Neacomys, Coendu, Proechymys and Marmosa - Annals and Magazine of Natural History Series 7 16: p308-314.
Trouessart EL 1898 - Catalogus Mammalium tam Viventium quam Fossilium. Fasciculus V: Sirenia, Cetacea, Edentata, Marsupialia, Allotheria, Monotremata - R.Friedländer & Sohn, Berolini.
Winge H 1893 - Jordfundne og Nulevende Pungdyr (Marsupialia) fra Lagoa Santa, Minas Geraes, Brasiliens - E. Museo Kundii Kjöbenhavn 2: p1-133.
ACKNOWLEDGEMENTS
Special thanks to Juan Carlos Chebez for providing important literature and Nilton Cáceres for very kindly reviewing texts and providing a copy of his book Os Marsupiais do Brasil.
MAP 30:
Micoureus paraguayanus


