

Phyllostomus hastatus (Pallas 1767) Image Gallery
TAXONOMY: Class Mammalia; Subclass Theria; Infraclass Metatheria; Order Chiroptera; Suborder Microchiroptera; Superfamily Noctilionoidea; Family Phyllostomidae, Subfamily Phyllostominae (López-Gonzalez 2005, Myers et al 2006). There are four species in this genus, two of which occur in Paraguay. The generic name Phyllostomus is Greek meaning “leaf mouth” in relation to the prominent nose leaf. The species name hastatus is Latin meaning “armed with a spear” again referring to the “spear-nose”.
No type specimen exists, the description being based on Buffon´s (1765) “La chauvesouris fer-de-lance”. Gardner (2007) recognises three subspecies, the nominate P.h.hastatus being present in Paraguay. Santos et al (2003) recognised only two, considered P.h.aruma a synonym of the nominate subspecies. Various other subspecies have been described mostly on the basis of perceived differences in size. Synonyms adapted from Gardner (2007) and López-González (2005):
V. [espertilio] hastatus Pallas 1767:7. Type locality "Amerique", restricted to “Surinam” by JA Allen (1904).
[Pteropus] hastatus Erxleben 1777:136. Name combination.
Phyllostomus hastatus Lacépède 1799:16 First use of current name combination.
Phyllostoma emarginata E. Geoffroy St.Hilaire 1803:60. Type locality "La Guiane”.
Phyllostoma hastatum E. Geoffroy St.Hilaire 1810:177. Name combination.
Phyllostomus maximus Wied-Neuwied 1821:242 Type locality "Die Wälderen an den Ufern des Rio das Contas”, Bahía, Brazil.
Phyllostomus hastatus panamensis JA.Allen 1904:233. Type locality "Boquerón, Chiriqui”, Panama.
Phyllostomus hastatus caurae JA.Allen 1904:234. Type locality "Cali, Upper Cauca Valley”, Colombia. Incorrect original spelling of invalid subspecies P.h.caucae (=P.h,panamensis)
Phyllostomus hastatus caucae JA.Allen 1916:225. Corrected spelling. Invalid subspecies (=P.h,panamensis)
Phyllostomus hastatus curaca Cabrera 1917:12. Invalid subspecies (=P.h,hastatus) type locality "Archidona sobre el Rio Napo”, Ecudaor.
Phyllostomus hastatus aruma O.Thomas 1924:236. Type locality "Taguatinga”, Toncantins, Brazil.
Phyllostomus hastatus paeze O.Thomas 1924:235. Invalid subspecies (=P.h,panamensis) type locality "Bogota”, Cundinamarca, Colombia.
ENGLISH COMMON NAMES: Great Spear-nosed Bat (Gardner 2007), Greater Spear-nosed Bat (Redford & Eisenberg 1992), Spear-nosed Bat (Santos et al 2003), Pallas Spear-nosed Bat (Goodwin & Greenhall 1961).
SPANISH COMMON NAMES: Murciélago que hoja nasal lanceolada (Emmons 1999), Murciélago nariz de lanza mayor (Aguirre 2007), Vampiro de lanza (Eisenberg & Redford 1999).
GUARANÍ COMMON NAMES: No known names.
DESCRIPTION: A huge, robust bat. Pelage short, smooth and velvety, with little difference between ventral and dorsal colouration. Across the range pelage colour varies from extremely dark brown or almost black, to more reddish, ranging through chestnut to almost orange (described as two colour phases by Lopez-González 2005, though there is a spectrum variation between the two extremes). Both Paraguayan specimens collected to date were reddish-brown. Nose leaf simple but well-developed with horseshoe free of the upper lip. Lower lip bears a V-shaped groove bordered by warty protuberances. Ears widely-separated, triangular and with pointed tips. Membranes, ears and facial skin black. Wing membrane attached to side of foot. Males with glandular chest sac. Tail short. Yearling females that have not yet bred are distinguishable from adults by their small, unpigmented nipples (Stern & Kunz 1998).
CRANIAL CHARACTERISTICS: Skull massive and robust with broad, low and flattened rostrum. Prominent sagittal crest. Goodwin & Greenhall (1981). Santos et al (2003) combined measurements published in the literature but did not distinguish between sexes, giving the following ranges: Greatest Skull Length 28.2-31.9mm (n=18); Transverse Zygomatic Width 14.6-16.3mm (n=18); Interorbital Constriction 6.7-7.4mm (n=18).
Anderson (1997) gives the following measurements for three males and one female from Bolivia: Condylobasal Length male 33-35.8mm female 33.4mm; Zygomatic Width male 19.1-21.2mm female 19.7mm; Lamboidal Width male 18.6-20.9mm female 18.6mm; Width of Braincase male 13.8-14.8mm female 13.8mm; Skull Depth male 14.3-15.8mm female 14.3mm; Width Across Canines male 9.2-10.4mm female 9.2mm.
The following measurements are for two Paraguayan specimens (a female and a male respectively) published in López-González (2005): Greatest Skull Length 36mm 37.7mm; Condylobasal Length 31.6mm 33.2mm; Transverse Zygomatic Width 18.9mm 20.4mm; Mastoid Width female 17.6mm; Interorbital Constriction 6.8mm 7.2mm; Width Across Upper Molars 12.5mm 13.2mm; Width Across Upper Canines 8.2mm 9.9mm.
DENTAL CHARACTERISTICS: I2/2 C 1/1 P2/2 M3/3 = 32. Deciduous teeth small and simple, permanent teeth robust and primitive. Posterointernal part of the molar crown with sharp cusps. McCracken & Bradbury (1981) report than males show less tooth wear than females but were unable to confirm whether this could be attributed to dietary differences or simply the fact that males have higher mortality than females.
Santos et al (2003) combined measurements published in the literature but did not distinguish between sexes, giving the following range: Upper Tooth Row 9.2-10.4mm (n=18). The following measurements are for two Paraguayan specimens (a female and a male respectively) published in López-González (2005): Upper Tooth Row 11.9mm 13.3mm; Lower Tooth Row female 14.1mm. Anderson (1997) gives the following ranges for three males and one female from Bolivia: Molar Width male 3.4-3.6mm female 3.3mm; Dental Span male 13.5-14.5mm female 13.4mm.
GENETIC CHARACTERISTICS 2n=32. FN=58. (Gardner 2007). X chromosome submetacentric, Y chromosome acrocentric. 2 rDNA sites and 2 teleometric chromosomes (Baker & Hsu 1970).
EXTERNAL MEASUREMENTS: A huge bat, the largest in Paraguay. There is evidence of sexual size dimorphism in certain cranial and external measurements with males being c 15% larger than females (McCracken & Bradbury 1981). Santos et al (2003) combined measurements published in the literature but did not distinguish between sexes, giving the following ranges: TL: 124-131mm (n=78); FA: 79-94mm (n=94); EA: 28-34mm (n=78); WT: 78-112mm (n=78). Giannini & Brenes (2001) gave the following masses for unsexed adults (n=10) from Costa Rica: WT: 107.4g (+/- 12.97g). Stern et al (1997) gave the following measurements for sexed adults from Trinidad: WT: male 89.1g (+/- 8.7g) female 76.3g (+/- 7.1g); WS: male 537mm (+/- 22mm) female 546mm (+/- 16mm).
Anderson (1997) gives the following measurements for one male and 15 females from Bolivia: HB: male 125mm, female 95-115mm; TA: male 25mm, female 21mm (n=1); FT: male 20mm, female 25-27mm; FA: male 86mm, female 78-87mm; EA: male 35mm, female 15-28mm; WT: female 72-110g.
McCracken & Bradbury (1981) gave the following weights for adults, yearlings and newborns in Trinidad: WT: adult male 94g (+/-6.8g, n=69) yearling male 92g (+/-6.6g, n=12); adult female 81.6g (+/-5.5g, n=161) yearling female 78g (+/-4.3g, n=11); WN: 13g. Stern & Kunz (1998) gave the following measurements for newborn bats (n=80) in Trinidad: FA: 34.1mm (+/-2.1mm) approximately 42% of mean adult length. Length of forearm increases linearly until the fifth week and reaches adult proportions by the 8th week; WN: 15.8g (+/-1.8g) approximately 20.7% of mean adult mass. Esbérard & Motta (2002) found two juvenile males of estimated age 15 to 20 days with the following measurements WT: 21g 40g; FA: 39mm 55.07mm.
The following measurements are for a single female Paraguayan specimen published in López-González (2005): TL: 115mm; TA: 22mm; FT: 19mm; FA: 79.1mm; EA: 28mm; WT: 91.1g. Additionally Baud (1981) gives the following measurements for a single male Paraguayan specimen: FA: 82.5mm.
SIMILAR SPECIES: This is a huge bat. The only other Paraguayan Phyllostomus, P.discolor is considerably smaller in all measurements. This species has a forearm in the range 78-87 mm, a head and body length of 95-115mm and mass typically >42g, compared to forearm of 56-58mm, head and body of 69-70mm and mass <39g in discolor. Note that the pelage does not extend onto the membranes in P.discolor, whereas in P.hastatus it extends onto the first third of the forearm and onto the uropatagium. Furthermore the tricoloured hairs of P.discolor give its pelage a grizzled effect dorsally and it is notably paler below compared to the overall more uniformly coloured P.hastatus. Structurally note that the calcar is as long as the hindfoot in this species, whereas in discolor it is shorter. Cranially the sagittal crest is well-developed. Dentally the first upper and lower incisors are higher than they are wide, whereas in discolor they are wider than they are high.
The only other bat approaching this species in size is Chrotopterus auritus which can be immediately identified on account of its exceptionally large ears (c48.3mm in C.auritus compared with c28mm in P.hastatus) and thick, woolly pelage, in contrast to the dense velvety pelage of this species. Note also that Chrotopterus has only a single pair of lower incisors whilst this species has two pairs.
DISTRIBUTION: Widely distributed through tropical lowland Central and South America. There are three recognised subspecies. The northernmost P.h.panamensis extends from southern Belize and Guatemala, south through Central America to Colombia, Ecuador and Peru west of the Andes, and north and west of Lake Maracaibo, Venezuela; P.h.aruma is known only from the type locality in Tocantins, Brazil. The nominate subspecies P.h.hastatus occupies the majority of the South American range from eastern Venezuela and the Guianas, south through Brazil to central Bolivia and northern Paraguay.
In Bolivia the species has been recorded in Departamentos Beni, La Paz, Cochabamba, Pando and Santa Cruz (Aguirre 2007). In Brazil the species is widespread and has been recorded in the following states: Acre, Amazonas, Amapá, Bahía, Ceará, Distrito Federal, Espirito Santo, Goiás, Maranhão, Minas Gerais, Mato Grosso do Sul, Mato Grosso, Pará, Pernambuco, Piauí, Paraná, Rio de Janeiro, Rondônia, Roraima and São Paulo (dos Reis et al 2007). There are no records from Argentina.
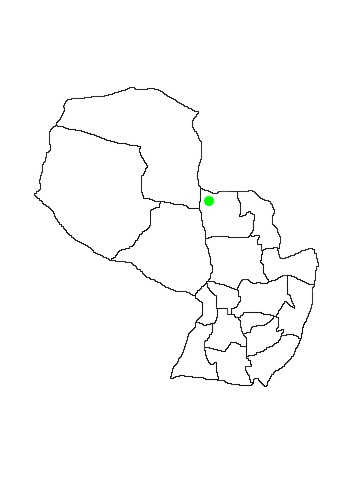
In Paraguay the species is known only from two localities in Departamento Concepción, “between Estancia Estrellas and Estancia Primavera” and at Estancia San Luís (López-Gonzalez 2005). The San Luís specimen RDO 1143 is an adult male deposited at Texas Tech University.
HABITAT: Widespread and with a catholic habitat preference, this species is distributed in lowland forested areas and nearby clearings. Tuttle (1970) captured the species on the edge of villages in Peru and they seem to tolerate human proximity where sufficient roosting and feeding opportunities exist and they are not persecuted. In fact they will even utilise palm-thatched roofs of dwellings for roosting and feed at fruiting trees in gardens. In Departamento Beni, Bolivia the species occurs in forest islands in the cerrado-like Beni savanna (Aguirre 2007), not dissimilar to the habitat in which the species has been collected in Paraguay. In the rest of Bolivia it is equally at home in humid forests and savannas as in the dry forests of the Chaco. In Manu National Park, Peru, Ascorra et al (1996) noted that this was the only locally occurring member of the subfamily Phyllostominae to regularly occur in disturbed areas.
In Paraguay all specimens have been collected in mixed tropical forest in palm savanna (López-Gonzalez 2005).
ALIMENTATION: Considered principally insectivorous by many authors, but the diet is supplemented by plant material, particularly nectar, fruit and flowers and in smaller quantities vertebrates such as birds, small bats and rodents. As a result it might best be considered an omnivore. The species has a powerful bite 68N (+/-1.99) generating more pressure than any bat yet measured and allowing it to consume large, hard-shelled prey (Aguirre et al 2003).
Foraging Behaviour and Diet Group foragers, larger groups gathering seasonally at particularly attractive resources. McCracken & Bradbury (1981) found that females had their own foraging area that they used throughout the year and that individual areas were clumped with those of other members of the same harem in a mean area of 66.5ha (+/-19ha).
Group foraging is considered to reduce search time and improve ability to defend resources from competitors but is more pronounced at times of year when resources are clumped. It was most prominent in the dry season in Trinidad when resources are scarce (Wilkinson & Boughman 1998) with a mean of 2.65 bats per group (+/-0.33, n=20 groups) at Ochroma trees in the dry season compared to 1.73 bats per group (+/-0.13, n=59 groups) at Cecropia trees in the wet season. It may also act to help inexperienced juveniles to locate isolated food resources. Females occasionally bring food back to the day roost which may serve to advertise the presence of abundant resources to other harem members. Typically females in medium-sized harems (14 to 17 individuals) foraged together more often than those in small or large harems and females in older groups foraged socially more often than females in newer groups. (McCracken & Bradbury 1981)
It is hypothesised that reciprocity may act in female harems with individuals advertising plentiful resources in their foraging areas to other members of the harem and in turn being “advised” of plentiful resources in the foraging areas of others. Such mutualism could arise given the long-term stability of these groups. (McCracken & Bradbury 1981). Competition between harem members for plentiful resources is extremely low, but rare, indivisible foods like large-bodied beetles may be hotly contested (Boughman 2006). Bachelor males however showed no such clumping and dispersed more widely to forage. Unstable, less structured bachelor groups, consisting of males potentially competing for access to females do not present the necessary conditions for such reciprocity to occur. They contain males of various ages and membership is constantly changing, creating the possibility that certain males could take advantage of others without reciprocating.
Kunz et al (1998) investigated the constraints on foraging males associated with harem maintenance. Females from the same roost typically forage in the same general area, with females from other harems having different foraging ranges. Harem males however forage closer to the roost due to the competing needs of foraging and harem defence. They found that harem females typically foraged only a few times per night for long periods, with their activity concentrated in the early part of the night. Harem males however foraged numerous times per night for shorter periods randomly throughout the night, returning to the roost when not foraging and engaging in harem defence activities. The following results were obtained from two different harems 1: Total foraging time harem male 93.3mins (+/-32.1mins) two females 145.8mins (+/-26.7mins); Number of foraging bouts harem male 6 (+/-2.6) two females 1.5 (+/-0.5); Mean duration of foraging bouts harem male 16mins (+/-1.5mins) two females 110mins (+/-49.4mins). 2: Total foraging time harem male 105mins (+/-21.2mins) four females 156.2mins (+/-45.1mins); Number of foraging bouts harem male 9mins (+/- 1.4) four females 2.6 (+/- 1.9); Mean duration of foraging bouts harem male 12mins (+/-4.2mins) four females 90.5mins (+/-59mins).
McCracken & Bradbury (1981) found that bachelor males spent more time foraging than either harem males or harem females. Adults left the harem to forage typically within 30 minutes of each other and returned within two hours of each other. They found no evidence of lunar phobia and that bats were equally active on moonlit nights as they were on cloudy nights. Furthermore bats were active during light rain, though very heavy rain delayed their departure from the roost or forced them to suspend foraging.
In the dry season in Bolivia the species feeds principally on large arthropods 15-25mm long, Scarabaeidae, Dytiscidae (both Coleoptera) and Gryllidae (Orthoptera) being the main groups taken in Departamento Beni. McCracken & Bradbury (1981) noted that in Trinidad they also take cockroaches (Dictyoptera), Passalidae (Coleoptera) and alate leafcutter ants Atta cephalotes (Hymenoptera). Bloedel (1955) noted groups feeding on swarming termites (Isoptera) around a flowering mango tree in Panama.
Groups of 10-20 were observed feeding on nectar of Ochroma lagopus in the Beni savannas (Aguirre 2007). Santos (2003) listed fruits of the following plant species as being consumed in Brazil: Achras sapota, Annona muricata, Artocarpus integrifolia, Carica papaya, Cecropia, Diospyros kaki, Eriobotrya japonica, Eugenia uniflora, Livistona chinensis, Lucuma caimito, Mangifera indica, Musa paradisiaca, Myrcia jaboticaba, Passiflora quadrangularis, Pilocarpus pinnatifolius, Psidium guajava, Rubachia glomerata, Solanum paniculatum, Terminalia catappa, and Vitis vinifera. In Belem, Brazil, it has been observed taking nectar from flowers of Ceiba pentandra, Parkia gigantocarpa, and P. pendula. Whilst in Trinidad & Tobago they take the fleshy funiculus of the Sapucaia nut Lecythis zabucajo (Goodwin and Greenhall 1961) and pollen of flowers of Hymenaea courbaril, with groups being observed foraging at both trees (McCracken & Bradbury 1981).
Silva & Peracchi (1995) suggest that flowering Pseudobombax grandifolium trees may represent an important food source for the species in winter when other food is in short supply. Greenhall (1966) records the species feeding on ripening Valencia oranges Citrus sinensis during the dry season in Trinidad, leaving circular holes and tooth marks in the fruit and consuming the pulp to a depth of “about one inch”. He adds that citrus fruits are usually avoided by fruit-eating bats. Furthermore an individual was also attracted to fruit that had been harvested and places in crates.
Wilkinson & Boughman (1998) noted significant seasonal variations in diet in Trinidad. From December to January most fecal pellets contained pollen of the balsa tree Ochroma lagopus, with the 2cm fruits of Rollinia multiflora being the second most common component. From April to June Cecropia peltata seeds and pulp predominated, with insects becoming more abundant by June. Seeds of cucumber Gurania spinulosa and Anguria angustifolia made up about 10% of the material but reduced in abundance from December to June. Amount of insect material was related to rainfall, with more insects consumed in the wetter months. These principal dietary components were consistent from year to year.
Oprea et al (2006) documented predation by this species on three species of bat Glossophaga soricina, Carollia perspicillata and Myotis nigricans in southern Brazil and suggested that the species may opportunistically prey on bats. All the victims were smaller species caught in mist nets. In the case of Glossophaga an individual of P.hastatus ate only the abdomen and then flew away. In the case of Carollia the net was close to a flowering tree and it was hypothesised that P.hastatus was feeding at the tree but opportunistically predated the smaller entangled bat, leaving only part of the left wing. On a third occasion a pregnant individual of Glossophaga was being consumed by one P.hastatus when another arrived, apparently also to feed, though it was driven away by the wing movements of the first. On the same night examples of Desmodus rotundus and Anoura caudifera were also found partly consumed, though the predating bat was not oberved. The authors suggested that carnivory was possibly more common than currently realised in this species and that given its abundance in the Atlantic Forest of Brazil that it might act to control bat populations.
Ascorra et al (1996) found no evidence of carnivory in Manu National Park, Peru and all fecal samples contained insect and plant remains. Seeds of the pioneer plant genus Cecropia sp were well-represented, as was the local fruit crop Pourouma cecropiaefolia. Pollen grains of both Musa paradisiaca and Ochroma pyramidale were recovered from the fur of this species. The species may play an important role in pollinisation and dispersal of some plants.
Diet in Captivity Giannini & Brenes (2001) studied the flower-feeding behaviour of this species under captive conditions in Costa Rica. Presented with banana inflorescences, the bats approached directly and landed head down, alternating feeding at numerous flowers with scanning of the surroundings. Total mean foraging time was 268 seconds (+/-133s) with the reaction time from being presented with the flowers 1200 seconds (+/-600s). The entire nectar supply was consumed and foraging efficiency (mean microlitres of consumed nectar per gram of mean body mass) was 17.4. Tuttle (1970) netted this species in Peru on the edge of villages wherever bananas where present. Netted individuals were frequently covered in pollen.
Dunn (1933) first documented the predatory behaviour of this species, though it had long been suspected of being “sanguineous”. He noted that seven bats of this species left overnight in a cage with 26 Carollia perspicillata azteca had completely devoured five individuals of the smaller species and killed all the others by morning. He presented captive individuals with fruit and occasionally pieces of meat, noting that the latter “was always quickly eaten”. A large female presented with a small dish containing 5cc of defibrinated blood had apparently emptied it by the next day, whilst the following night it “drank another 5cc of blood, ate a good sized piece of monkey liver and also some banana”. The same individual also devoured ten large cockroaches Periplaneta americana in a single night, hanging from the roof of the cage and holding the insect with the “thumbs and ends of the forearms”, making a crunching and snapping noise that would “lead one to suspect she was deriving keen pleasure from her lunch”.
Adult house mice Mus musculus were also consumed, typically the head and forequarters being eaten and the hindquarters and tail discarded. On one occasion the bat was seen to grip the mouse in front of the hips with the mouth and to hold it with the wings whilst hanging by a single foot from the roof of the cage; the mouse being still alive, struggling and attempting to bite the bat. On several occasions the bat tried to hold the head of the mouse with its thumbs to shift its grip, eventually being forced to place the head inside the wings (evidently using them to help manipulate her grip) and emerging gripping the mouse in the mouth by the back of the neck having apparently killed it in the process. The mouse was consumed head first, the wings of the bat being partly extended in front of the venter with the ends of the forearms held together and the second and third metacarpals of one wing held together and in close contact with those of the other wing to form a pocket which helped support the mouse carcass. The head, shoulders and breast were consumed within 16 minutes and the rest of the carcass was allowed to drop to the floor, though six hours or so later she descended to the floor and finished the meal leaving “only the tail and a few small pieces of entrails”. Another mouse that evaded capture and took refuge in a piece of cloth on the cage floor was actively pursued by the bat, which placed its head under the cloth and retrieved the mouse, holding it in the mouth by the back of the neck.
When presented with a blue honeycreeper Cyanerpes cyaneus the female bat was unable to capture it during the day, despite making several attempts to drop on it from the side wall of the cage. It did however successfully capture and consume the bird during the following night, devouring even the beak, claws and all but the largest feathers. The same bat also consumed a house wren Troglodytes aedon and other small bats it was presented with including Carollia perspicillata, Uroderma bilobatum, Molossus coibensis and Glossophaga soricina. Prior to its death, the latter showed no fear of the larger bat and hung from the cage roof six inches from its eventual assassin. More surprising was the consumption of a male Noctilio albiventris, a large bat with an offensive musky odour, though the initial approach was tentative. The head (except the teeth and mandible), abdomen and entrails were consumed, but the thorax and abdomen were left untouched, in the author´s opinion due to the presence of a “soft, oily, wax-like substance” that had a “very objectionable odour”. Another Noctilio was pursued for over an hour with half-hearted snappy bites which the author interpreted as an attempt to wear out the victim and perhaps disable it with a single snap.
On each occasion the bat fed whilst hanging from the roof of the cage. Bones were consumed but in fecal pellets the only animal remains that were noticeable were hairs. Typically the meals would be half consumed and then dropped, only to be retrieved six or seven hours later and “finished”. It was considered unlikely that a bat would retrieve a dropped carcass under wild conditions and estimated that two or three prey items could be killed during the course of a night in the wild. Providing the bat with multiple prey items resulting in the killing and devouring of all. However this theory does not allow for the fact that other, more plentiful and more readily available food sources are also available under wild conditions.
Esbérard & Motta (2002) raised captive juveniles from the age of 15 to 20 days using powdered Nestogeno milk, providing 1-2ml by syringe every two hours for the first week and maintaining a temperature of 31ºC. From 16 to 40 a 50% solution of Nestogeno and full fat cream was used and from 41-60 days a solution of equal parts Nestogeno, full fat cream and soymilk was provided. From 75 days onwards they were switched to a mixture of 75% mashed fruit (mainly pineapple), 15% Psitamixa (a commercial parrot diet supplement), 5% minced meat, 5% puppy mix 2.5% egg yolk and 0.5% honey - the same mix being offered successfully to breeding adults.
REPRODUCTIVE BIOLOGY: Harem breeders, a single male maintains numerous females and adopts a female-defence polygamy breeding strategy (Kunz et al 1998). No breeding data specifically for Paraguay is available.
Seasonality Monoestrous in Central America but polyoestrous through much of South America. In the Brazilian cerrado breeding has been documented from April to August with lactating females captured from September to April (Willig 1985). In Peru twelve pregnant females with embryos of mean CL length 27.4mm (range 22-30mm) were found in August as well as 8 non-pregnant females (Tuttle 1970). A pregnant female was taken in July in Colombia (Arata & Vaughn 1970). In Venezuela one of seven specimens captured in April was lactating (August & Baker 1982).
In Trinidad & Tobago reproduction is synchronised from March to September with young being born in the dry season and weaned early in the rainy season. Copulations took place from late October to December and by March all mating activity had ceased. Birthing took place in the space of a few weeks from mid-April to mid-May. (McCracken & Bradbury 1981). Porter & Wilkinson (2001) found that birth dates varied annually over four years of observations in Trinidad and that environmental factors could be used to explain only 40% of the variation. Mean parturition date varied annually and differed significantly between colonies and between groups within a single colony. Colony cave was estimated to be responsible for 39% of the variation in birth dates in the years 1990-1991 and group differences accounted for 19% of the variation. Rainfall patterns were considered to be the primary environmental factor affecting reproduction timing.
Social cues were also found to affect the timing of breeding. Under experimental captive conditions and deprived of environmental clues two groups initially showed less synchrony than wild groups, but females moved between groups gave birth in synchrony with their new groups and out of synchronisation with their former group. It was hypothesised that such social clues were actively transferred by females within a group or else imposed by the mating schedule of the single attendant male, possibly via secretions from the sternal gland. (Porter & Wilkinson 2001)
Courtship As with other members of the genus males possess a chest gland which secretes a strong smelling fluid. Males rub this fluid over females in their harem to give the group a characteristic odour. This olfactory clue may also be used in addition to group-specific vocalisations to help group members to identify each other. (Boughman 1997). Within any one colony females greatly outnumber males anywhere in the order of 2:1 to 10:1, with a mean of 2.8:1, and it was hypothesised that the bias was caused by greater mortality of males. (McCracken & Bradbury 1981).
Harem males actively defend their access to females as roosting “bachelor groups” form close by. Males in the bachelor groups will attempt to steal matings from harem females during times when the harem male is absent. Random foraging patterns by harem males were considered to assist in roost defence by Kunz et al (1998); a predictable foraging pattern would enable bachelor males to more easily exploit the absence of the harem male for chance matings with females.
Typically harem males maintained their females for several years and were usually older than bachelor males. Males that lost control of their harem typically did not regain it, with McCracken & Bradbury (1981) finding that only 1 male of 10 that lost control of its harem later regained it. Males never moved between harems. Boughman (2006) found harems in Trinidad to consist of 10 to 22 females with a mean of 17. McCracken & Bradbury (1981) gave a mean of 17.9 (+/-5.1, range 7-25). However the two smallest harem groups recorded were the result of disturbance during sampling and if excluded from consideration the mean group size rose to 19.5 (+/-3.1).
Of 48 adult females marked in the same study in Trinidad, 87% were in the same harem after 12 months and only five permanently switched groups, all other movements being temporary (McCracken & Bradbury 1981). Harem females have an average relatedness to one another of almost zero (McCracken & Bradbury 1977) but paternal half-sisters will often join a new harem together (Boughman 1997). New harems form from groups of yearling females drawn from different harems and colonies, and incidences of young females joining established harems are apparently rare. As a result harems typically consist of females of a similar age, with young groups, middle-aged groups and older groups. Experimentally disrupted harems reformed, even in the absence of harem males. (McCracken & Bradbury 1981).
Kunz et al (1998) did not consider the presence of subadult males within the harem as a threaten to the harem male. It was even suggested that these subordinate males benefitted from the close proximity to the females in the harem, standing them in better stead to take over the harem should the harem male die or be displaced.
MAP 36:
Phyllostomus hastatus
Pregnancy Birthing is highly-synchronised within a harem over a 2 or 3 week period and each female gives birth to a single young. The gestation period is five months (Porter & Wilkinson 2001). In a colony in Trinidad 219 females were nursing young, 7 were still pregnant and only 11 mature females showed no sign of reproductive activity during this period. Males constantly check receptivity of females during the breeding period. Boughman (2006) found that maternal condition differed greatly between harems in a single colony in Trinidad and that females in older groups gave birth sooner than those in younger groups.
Sex ratio of juveniles at birth did not differ significantly from 1:1 and the harem male could be demonstrated to be the father of the offspring born into his harem in 85-100% of cases in 8 sampled harems. Given a mean harem size of 19 females and the fact that some males may hold territories for up to three years, it was estimated that the most successful males may father more than 50 offspring in their life time, whilst bachelor males that never gain control of a harem may never reproduce. In one of the harems sampled it was found that the harem male was not the father of the majority of the offspring produced by his harem females. However this could be explained by the fact that the male took control of the harem during the mating period and the previous harem male was in fact the father of the majority of the young born. The fact that the harem male could not claim paternity of the offspring did not lessen his defence of the harem, and similarly there was no attempt to abort the foetuses by females. (McCracken & Bradbury 1981).
The fact that a single male could father such large numbers of offspring has obvious potential genetic consequences. However the highly structured social units consisting of unrelated individuals combined with the fact that juveniles disperse widely help to prevent bottlenecking and the spread of rare genes through the population (McCracken & Bradbury 1981).
Development Juveniles are carried on foraging flights with their mothers for the first few days, but are later left unattended in the roost. Until about 4 weeks of age the juvenile is attached to the breast whenever the female is at the roost. Males are typically heavier than females at birth and gain mass at a faster rate, but there is no significant difference in forearm length between the sexes at birth. Forearm length and body mass of both sexes increase linearly from birth before reaching asymptotes. Forearm length was found to be a good predictor of age for juvenile bats (Stern & Kunz 1998). Boughman (2006) found no evidence of sex bias in maternal investment and that maternal condition had no discernible effect on the early condition of pups.
Juveniles occasionally fall to the cave floor and are frequently retrieved by the mother. McCracken & Bradbury (1981) stated that babies are tended only by their mother and no female was seen attending another infant during their comprehensive study. A later study by Bohn et al (2009) also in Trinidad found that in fact pup-guarding by adults other than the mother was commonplace. They calculated that 4% (+/-2%) of the pups in their study group fell in their first week of life.
Fallen pups are agile on the floor, typically flapping their wings and scampering up the cave wall to a height of 1 or 2m from where they produce audible isolation calls and wait to be retrieved. Such calls attract the attention of numerous females, with a mean of 17 adults (+/-2.6) visiting fallen juveniles that called in this manner, and one pup receiving a maximum of 342 visits without being retrieved. No banded males were seen to visit pups. Such visits were the only times that adults where seen on the cave floor or walls in a total of 200 hours of observations. The majority of visits (60%) were short 4.2s (+/-0.3s), involving a female landing at the pup´s side, briefly investigating it and departing again. Females visited pups from their own harem more frequently (61% of occasions) and for longer periods 32.2s (+/-5s) than they did to pups from other harems 17.5s (+/-0.74s). (Bohn et al 2009).
Behaviour of females visiting fallen pups differed depending on whether the visiting adult was the pup´s mother, from the mother´s harem or from a different harem. Mother´s invariably landed beside the pup, lifted a wing and allowed the pup to attach to a nipple before returning it to the harem. On only 6 of 63 visits did a female incorrectly identify a fallen pup as her own, and in 3 of those cases the female was temporarily missing her own pup. Females from other harems bit the head of the pup (sometimes fatally) and then either departed or carried the pup away in its mouth, five of the eight pups which suffered this latter fate later being found dead outside the colony cave. Bitten pups often produced distinctive loud vocalisations. Females from the same harem would visit pups in response to aggression from alien females and such acts appeared to decrease the likelihood of the pup then being carried away and killed. On such occasions they would leave their own pup unprotected in the harem.
Male pups were attacked with more frequency than females, with 20 of 27 bitten pups being male and 29 of 61 unbitten pups being male. It was considered that this may be indicative of sexual selection, as the breeding system means that few males breed, the preferential killing of male pups increases the possibility of breeding success for the sons of the attacking female. Furthermore when pups are present in the harem at least one adult female usually remains in the colony whilst the others depart to feed, presumably acting to communally protect the pups when they would otherwise be vulnerable to attack (Boughman & Wilkinson 1998).
Stern et al (1997b) studied the dynamics of lactation in the species. They found that fat and dry matter of milk increased from 9 to 21% during lactation and from 21 to 35% of wet mass. As a result of the increased fat concentration energy also increased from 6 to 9kJ/g-1. Total sugar levels showed a minor decrease and mean sugar level was 3.96% (+/-1.07%) of wet mass. Protein concentration increased from 6 to 11% of wet mass during the peak of lactation then steadily declined again towards weaning. Total milk energy output when pups began to forage (day 49) was 3609kJ. The following mineral levels were present as mg/g-1 of dry matter: Magnesium 0.55mg/g-1 (+/-0.26mg/g-1); Iron 0.23mg/g-1 (+/-0.2mg/g-1); Calcium 8.75mg/g-1 (+/-4.17mg/g-1); Potassium 5.42mg/g-1 (+/-2.11mg/g-1); Sodium 9.87mg/g-1 (+/-4.3 mg/g-1). Mean water content of milk to day 49 was 73.4%. Period of lactation varied between individuals, some females stopping lactation at 92 days and others continuing at 101 days. Milk production was not considered an accurate indicator of weaning as one female which lost her pup continued to produce milk for up to 4 weeks afterwards.
Stern et al (1997) studied the ontogeny of flight in juvenile bats of this species. They found that suckling bats first take flight at 43-54 days (7-8 weeks), with about 50% of the sucklings being volant by the end of week 7 and all volant by the end of week 8. Wing area and wingspan increased linearly, and wing loading decreased, through week 8. Aspect ratio continued to increase until week 9. Mean body mass at first flight was 59.1g, or 78% the mean body mass of adults. Wing loading of males (76.2g +/-4.1g) and females (67.5g +/-6.3 g) also differed at week 8 as males had a greater mass. Body mass continued to increase for the first 100 days at which stage some of the phalanges were still cartilaginous and wing area, wing loading, aspect ratio, and wingspan were all considerably less than those of adults. No correspondence between emergence time from the roost of juveniles and mothers was found, suggesting that the role of flight tutoring by the mother is at best minimal. Radio-tagging revealed an increase in the distance and duration of foraging bouts made by young bats as they gained flight experience.
Juvenile bats are initially inept fliers and Stern & Kunz (1998) recorded hearing crashing noises in foliage and then finding juvenile bats that had apparently crash-landed. It was suspected that mortality during this period would be high. Dispersal of young following maturity is little known. Some bats remain at the natal colony for years after weaning (Stern & Kunz 1998) but McCracken & Bradbury (1981) noted that juveniles of both sexes dispersed and were not recruited into parental social units. Sexual maturity is reached at 16 months (Aguirre 2007).
GENERAL BEHAVIOUR: Unknown in Paraguay, though this is a widespread and often common bat that has been well-studied elsewhere.
Activity Levels Aguirre (2007) notes that the species is active in savannas in Departamento Beni, Bolivia from 6-10pm, whereas in Cochabamba, Selaya (2001) found it active from 6pm to 2am with a peak of activity between 8 and 10pm.
Flight Pattern This is a large bat with a direct flight style. When compared to other Phyllostomids this species has relatively long wings (Santos et al 2003). Stern et al (1997) gave the following measurements associated with flight for sexed adults: Wing area male 424.8cm2 (+/-26.8cm2) female 434cm2 (+/-25.1cm2); Aspect ratio male 6.7cm2 (+/-0.4cm2) female 6.9cm2 (+/-0.3cm2); Wing loading male 20.4Nm2 (+/-2Nm2) female 17.4Nm2 (+/-1.8Nm2) Wing loading of females fluctuates with changes in body mass due to available food and reproductive condition. Mean body mass of females and corresponding wing loading in Trinidad varied as follows throughout the year: In January when food is scarce WT: 72.2g (+/- 4.5g) Wing loading 16.3Nm2; In late pregnancy WT: 88g (+/- 5.1g) Wing loading 19.9Nm2; Postpartum WT: 75.4g (+/- 5.8g) Wing loading 17.1Nm2.
Giannini & Brenes (2001) gave the following measurements associated with flight for unsexed adults (n=10) from Costa Rica: Wing loading 19.2Nm2 (+/-2.25Nm2); Aspect ratio (wingspan/area of wings and chest) 7.6 (+/-0.25); Tip area index (area of dactylopatagium or wing tip area between the fingers/area of wing) 0.7 (+/-0.04); Tip length index (length of Digit III/length of arm and forearm 1.35 (+/-0.06); Tip shape index (tip area index/tip length index - tip area index) 1.2 (+/-0.06).
Roosts High fidelity to roost sites facilitates recapture of marked individuals (Stern et al 1997). Typically females form stable roosts which may stay together for 16 years or more and are then competed for by harem males, the winner gaining preferential access to them. McCracken & Bradbury (1981) hypothesised that such all female roosts arose from active co-operation of females on the feeding grounds. Colonies may consist of numerous distinct harem groups, with more unstable groups of non-harem males and subadults frequently roosting close by, but deprived of direct access to the females (Kunz et al 1998). Position of harems within a colony is also stable from year to year (McCracken & Bradbury 1981). Though individuals may move between groups in a single colony, during five years of observations not one individual was observed to move between colonies, despite other colonies being well within flight range (Boughman & Wilkinson 1998).
Bachelor groups are more variable in size than harems, consisting of 4 to 34 males (mean 17.7, +/-11). Typically bachelor groups are dominated by males, but a small number of pre-reproductive females with unused mammae are often present at a ratio of approximately 1:7 males, suggesting that females may temporarily reside in such groups prior to joining harems. Membership of bachelor groups is more unstable than that of harems, with males leaving as they acquire harems and the whole group responding to major disturbances by disbanding, relocating or abandoning a colony. Approximately 59% of males in a bachelor group were present in the same bachelor group 12 months later. (McCracken & Bradbury 1981).
Tuttle (1970) mentions colonies of 10 to 100 individuals in Peru, with size depending on the available space. Roosts were found in hollow trees, caves, termite nests and thatched roofs and at least one individual of this species was found in every roost containing Lophostoma silvicolum. In Bolivia Aguirre (2007) notes groups of 8 to 10 in active termite nests and the use of holes in the palm Copernicia alba (a species common in Paraguay) as well as a colony of approximately 250 individuals in a cave in Departamento Cochabamba during February.
Goodwin & Greenhall (1981) considered it bold at the roost and that considerable effort was required to disturb colonies. The following species of bat that occur in Paraguay were occasionally associated with roosts of this species in Trinidad: Carollia perspicillata, Desmodus rotundus, and Molossus rufus (=ater). Bloedel (1955) noted roosts in Panama under bridges, in a church and in a shed and that the species always hung from the highest areas available, with other species often occupying lower roost sites. He commented on the fact that other bats showed no apparent fear of this species despite its demonstrated predatory habits of small bats in captivity. Ascorra et al (1996) mention additionally roosts under large, dry palm leaves in Manu National Park, Peru.
Grooming Behaviour Adults in Trinidad collected from 2 to 18 August showed no signs of moult (Carter et al 1981).
Aggressive Behaviour Harem males aggressively defend their harems from other males by moving to the periphery of their harem, vocalising and wing beating. If necessary the invading males were attacked and bitten, with fights always been rapid, violent and decisive, invariably resulting with the retreat of the intruder. (McCracken & Bradbury 1981). Goodwin & Greenhall (1961) note that an individual in a cluster advanced and bit at a gloved hand, with McCracken & Bradbury (1981) speculating that this was likely the male exhibiting harem defence behaviour. Alternatively males show no response to the movement of females, which apparently can leave or join harems at will.
Mortality Mortality of juveniles was highest at birth and during weaning. About 40% of juveniles do not survive to independence (Stern 1996) and 25% do not make it to their first flight (Bohn et al 2009). Boughman (2006) found considerable variation in mortality of young between harems in a single colony, with 100% of young surviving to independence in one colony, while only 15% survived in the harem with highest mortality. Stern & Kunz (1998) found no association between mass of juveniles at birth or forearm length and mortality. McCracken & Bradbury (1981) estimated that population would remain stable if one in four female young survived to reproduce.
In Trinidad juveniles have been taken by opossums Didelphis marsupialis, tegu lizards Tupinambis sp., bullfrogs Rana catesbeiana and screech-owls Megascops choliba. (Boughman 2006).
Parasites Goodwin & Greenhall (1981) list the following parasites of this species in Trinidad: batflies Euctenodes mirabilis, Trichobius mixtus and T.dugesii (all Streblidae) and unidentified trematodes. Boughman (2006) found mean parasite load of Streblid flies (Trichobius sp. and Euctenodes mirabilis) on juveniles in Trinidad to be 7.1 per pup (range 2-14.4).
Longevity This is a long-lived species with low adult mortality (annual survivorship estimated at over 90% for females) and females may live for up to 20 years in the wild (McCracken & Bradbury 1981).
VOCALISATIONS: Three broad call types are known in this species, screech calls associated with group-foraging (6 to 11kHz), isolation calls produced by juveniles separated from their parents (15 to 100kHz) and low amplitude, short duration, frequency-modulated echolocation calls used for orientation (80 to 40kHz).
Boughman (1997) demonstrated that members of a harem that forage together share group specific calls that favour group recognition rather than individual recognition. Inter-group differences are defined by variations in at least nine characters, with pulse duration being the most significant. Calls from groups within a single cave colony were closer to each other than those from other caves, but each individual harem also differed within a single colony. However no evidence was found that calls identified individuals within a single group. It is likely that group identifying calls would be favoured over individual identifying calls in a group living species that forages in large aggregates (Boughman & Wilkinson 1998). Noisy, sudden onset, screech calls are easily located and carry far enough to reach other group members that may be some distance away. Bohn et al (2004) estimated that these calls could travel distances of between 70 and 109m at frequencies of 5 and 10kHz respectively.
Wilkinson & Boughman (1998) noted that seasonal differences in diet influenced screech calling and that females called more and foraged in larger groups when feeding on concentrated rather than dispersed resources. As a result calling was more frequent in the dry season (when food resources are scarce) than in the wet season. Screech calls occurred more often when bats flew in groups than when they flew alone, and calling females were joined by group members more often than were non-calling bats. Screech calls were rarely heard from males (consistent with their more solitary feeding behaviour) and were not heard when females were returning to the cave, only on departure. Boughman (1997) notes that bats leaving a roost in Trinidad initially flew below the canopy at a height of 3 to 20m, but on departing for the foraging sites they then flew above the canopy level. On leaving the roost screech calls often attracted other individuals that flew close behind (at a distance of c1m), possibly using smell to further identify the caller. Wilkinson & Boughman (1998) considered that the primary role of the screech call was contact which aimed to recruit and coordinate foraging of group members.
Group members are unrelated and Boughman (1998) demonstrated that screech calls were learnt and subject to change as group composition changed. Bats moving between groups initially differed in their call structure in seven variables but after five months of residency with the new group they differed in only one variable. The time taken to “learn” group calls was considered important in avoiding “cheating”, ie an individual imitating the calls of another group to take temporary advantage.
There is no detectable difference in individual calls between established members of the same group, though calls decrease slightly in frequency as bats age. Younger bats call more than older bats. Loud broadband screech calls are in the range 4-18kHz. Maximum energy in pulses is centred around 6725Hz (+/-36.3Hz) with a second energy peak at 8822Hz (+/-63.5Hz). Some pulses may have more than two energy peaks with an average of 2.2 (+/-0.3) peaks per pulse. Energy is distributed broadly across frequency. The bandwidth is 6838Hz (+/-74.6Hz), beginning at 4700Hz (+/-24.9Hz) and reaching to 11,537Hz (+/-68.9Hz). Amplitude increases in the approach to the first peak (136Hz/db +/-2.3Hz/db), more rapidly than is the decrease from the second 185Hz/db (+/-3.6Hz/db). Calls have a mean of 4.2 (+/-0.2) pulses each and last 1065ms (+/-59ms). Mean pulse duration is 229ms (+/-7ms) and pulses are given in quick succession having little or no interval between them.
Juveniles that fall from the harem give isolation calls. Such calls are variable and composed of different numbers of notes, though double-note calls are the simplest and most frequently produced. Bohn et al (2007) studied these calls and found that they were individually specific and sufficent for the mother to recognise her own pup. Spectral rather than temporal acoustic cues were more important for individual recognition. Isolation calls of pups from the same harem were closer to each other than they were to those of pups of other harems. It was suggested that this was indicative of the fact that the calls were heritable. Pup calls were not affected by sex but were affected by age, becoming shorter and increasing in frequency as the pup ages. Isolation calls were considered essential to the survival of pups as those that are not retrieved inevitably die. Bohn et al (2004) found that selection for offspring recognition exerts a strong influence on the sensory system of this species given that social living leads to an acoustically cluttered environment.
HUMAN IMPACT: None in Paraguay where it is a little known species with an apparently restricted range. Elsewhere this species is common, large and conspicuous, and the fact that it tolerates human presence has led to inevitable local legends. One of the most common is that this species is a vampire and in some areas it is likely persecuted as a result (Elliott 1904).
CONSERVATION STATUS: Globally considered to be of Low Risk Least Concern by the IUCN, see http://www.iucnredlist.org/details/17218/0 for the latest assessment of the species. The species is widespread and even abundant in some areas and able to tolerate considerable human disturbance. Though the Paraguayan population is at the southern extreme of its known range, it would not appear to be under any immediate threat and is likely stable. The species is best considered Data Deficient at national level, but may prove to be more widespread than currently thought.
CITE AS: Smith P (2009) FAUNA Paraguay Online Handbook of Paraguayan Fauna Mammal Species Account 36 Phyllostomus hastatus.
LAST UPDATED: 30 July 2009.
REFERENCES:
Aguirre LF 2007 - Historia Natural, Distribución y Conservación de los Murciélagos de Bolivia - Fundación Simón I. Patiño, Santa Cruz.
Aguirre LF, Herrel A, Van Damme R, Matthysen E 2003 - Implications of Food Hardness to Trophic Niche Partitioning in a Neotropical Savanna Bat Community - Journal of Functional Ecology 17: p201-212.
Allen JA 1904 - New Bats from Tropical America with Note on Species of Otopterus - Bulletin AMNH 20: p227-237.
Allen JA 1916 - List of Mammals Collected in Colombia by the American Museum of Natural History Explorations 1910-1915 - Bulletin AMNH 35: p191-238.
Anderson S 1997 - Mammals of Bolivia Taxonomy and Distribution - Bulletin AMNH 231.
Arata AA, Vaughn JB 1970 - Analyses of the Relative Abundance and Reproductive Activity of Bats in Southwestern Colombia - Caldasia 10: p517-528.
Ascorra CF, Solari S, Wilson DE 1996 - Diversidad y Ecologia de los Quirópteros en la Pakitza in Wilson DE, Sandoval A eds Manu: The Biodiversity of Southereastern Peru - Editorial Horizonte, Lima.
August PV, Baker RJ 1982 - Observations on the Reproductive Ecology of Some Neotropical Bats - Mammalia 46: p177-181.
Baker RJ, Hsu TC 1970 - Further Studies on the Sex-chromosome Systems of the American Leaf-nosed Bats (Chiroptera: Phyllostomatidae) - Cytogenetics 9: p131-138.
Bloedel P 1955 - Observations on the Life Histories of Panama Bats - Journal of Mammalogy 36: p232-235.
Bohn KM, Boughman JW, Wilkinson GS, Moss CF 2004 - Auditory Sensitivity and Frequency Selectivity in Greater Spear-nosed Bats Suggest Specializations for Acoustic Communication - Journal of Comparative Physiology, A 190: p185-192
Bohn KM, Wilkinson GS, Moss CF 2007 - Discrimination of Infant Isolation Calls by Female Greater Spear-nosed Bats, Phyllostomus hastatus - Animal Behaviour 73: p423-432.
Boughman JW 1997 - Greater Spear-nosed Bats Give Group-distinctive Calls - Behavioural Ecology and Sociobiology 40: p61-70.
Boughman JW 1998 - Vocal Learning by Greater Spear-nosed Bats - Proceedings of the Royal Society of London B 265: p227-233.
Boughman JW 2006 - Selection on Social Traits in Greater Spear-nosed Bats Phyllostomus hastatus - Behavioural Ecology and Sociobiology 60: p766-777.
Boughman JW, Wilkinson GS 1998 - Greater Spear-nosed Bats Discriminate Group Mates by Vocalizations - Animal Behaviour 55: p1717-1732
Boughman JW, Wilkinson GS, Moss CF 2009 - Pup Guarding by Greater Spear-nosed Bats - Behavioural Ecology and Sociobiology online.
Buffon G 1775 - Histoire Naturelle, Générale et Particulière Avec la Description du Cabinet du Roi - L´Imprimiere Royale, Paris 13.
Cabrera A 1917 - Mamíferos del Viaje del Pacífico Verificado de 1862-1865 por una Comisión de Naturalistas Enviada por el Gobierno Español - Trabajos del Museo Nacional de Ciencias Naturales Serie Zoologia, Madrid 31: p1-62.
Carter CH, Genoways HH, Loregnard RS, Baker RJ 1981 - Observations on Bats from Trinidad with a Checklist of Species Occurring on the Island - Occasional Papers of the Museum of Texas Tech University 72.
Dunn LH 1933 - Observations on the Carnivorous Habitats of the Spear-nosed Bat Phyllostomus hastatus panamensis Allen in Panama - Journal of Mammalogy 14: p188-199.
Eisenberg JF, Redford KH 1999 - Mammals of the Neotropics: Volume 3 The Central Neotropics - University of Chicago Press, Chicago.
Elliott DG 1904 - The Land and Sea Mammals of Middle America and the West Indies - Field Columbian Museum Zoology Series 4.
Emmons LH 1999 - Mamíferos de los Bosques Húmedos de América Tropical - Editorial FAN, Santa Cruz.
Erxleben JCP 1777 - Systema Regni Animalis per Classes, Ordines, Genera, Species, Varietates cum Synonymia et Historia Animalium. Classis 1 Mammalia - Lipisiae: Impensis Weygandianis.
Esbérard C, Motta AG 2002 - Recria Artificial de Falso-vampiro Phyllostomus hastatus - Chiroptera Neotropical 8: p152-155.
Gardner AL 2007 - Mammals of South America Vol 1: Marsupials, Xenarthrans, Bats and Shrews - University of Chicago Press, Chicago.
Geoffroy St.Hilaire E 1803 - Catalogue des Mammifères du Muséum National d´Histoire Naturelle - Paris.
Geoffroy St.Hilaire E 1810 - Sur les Phyllostomes et les Mégadermes Deux Genres de la Famille de Chouve-souris - Ann. Museu Histoire Naturelle Paris 15: p86-108.
Giannini NP, Brenes FV 2001 - Flight Cage Observations of Foraging Mode in Phyllostomus discolor, P.hastatus and Glossophaga commissarisi - Biotropica 33: p536-550.
Goodwin GG, Greenhall AM 1961 - A Review of the Bats of Trinidad and Tobago: Descriptions, Rabies Infection and Ecology - Bulletin AMNH 122: p187-302.
Greenhall AM 1966 - Oranges Eaten by Spear-nosed Bats - Journal of Mammalogy 47: p125.
Kunz TH, Robson SK, Nagy KA 1998 - Economy of Harem Maintenance in the Greater Spear-nosed Bat Phyllostomus hastatus - Journal of Mammalogy 79: p631-642.
Lacépède BGE 1799 - Tableau des Divisions, Sous-divisions, Ordres et Genres des Mammifères - Plassan, Paris.
López-Gonzalez C 2005 - Murciélagos del Paraguay - Biosfera Numero 9.
McCracken GF, Bradbury JW 1977 - Paternity and Genetic Heterogeneity in the Polygynous Bat Phyllostomus hastatus - Science 198: p303-306.
McCracken GF, Bradbury JW 1981 - Social Organization and Kinship in the Polygynous Bat Phyllostomus hastatus - Behavioural Ecology and Sociobiology 8: p11-34.
Oprea M, Vieira TV, Pimenta VT, Mendes P, Brito D, Ditchfield AD, de Knegt LV, Esbérard CEL 2006 - Bat Predation by Phyllostomus hastatus - Chiroptera Neotropical 12: p255-258.
Pallas PS 1767 - Vespertiliones in Genre in Spicilegia Zoologica Quibus Novae et Obscurae Animalium Species Iconibus, Descriptionibus Atque Commentariis Illustrantur - GA Lange, Berolini.
Porter T, Wilkinson GS 2001 - Birth Synchrony in Greater Spear-nosed Bats, Phyllostomus hastatus - Journal of Zoology, London 253: p383-390.
Redford KH, Eisenberg JF 1992 - Mammals of the Neotropics: Volume 2 The Southern Cone - University of Chicago Press, Chicago.
Reis dos NR, Peracchi AL, Pedro WA, Lima de IP 2007 - Morcegos do Brasil - Londrina, Brazil.
Santos M, Aguirre LF, Vázquez LB, Ortega J 2003 - Phyllostomus hastatus - Mammalian Species 722: p1-6.
Silva SSP, Peracchi AL 1995 - Observação da visita de morcegos (Chiroptera) as flores de Pseudobombax grandifolium (CAV.) A. Robyns. - Revista Brasileira de Zoologia 12: p859-865.
Stern AA 1986 - Growth and Reproductive Energetics of the Greater Spear-nosed Bat Phyllostomus hastatus - PhD Thesis University of Boston.
Stern AA, Kunz TH 1998 - Intraspecific Variation in Postnatal Growth in the Greater Spear-nosed Bat - Journal of Mammalogy 79: p755-763.
Stern AA, Kunz TH, Bhatt SS 1997 - Seasonal Wing Loading and Otogeny of Flight in Phyllostomus hastatus (Chiroptera: Phyllostomidae) - Journal of Mammalogy 78: p1199-1209.
Stern AA, Kunz TH, Studier EH, Oftedal OT 1997b - Milk Composition and Lactational Output in the Greater Spear-nosed Bat Phyllostomus hastatus - Journal of Comparative Physiology B 167: p389-398.
Thomas O 1924 - New South American Small Mammals - Annals and Magazine of Natural History Series 9 13: p234-237.
Tuttle MD 1970 - Distribution and Zoogeography of Peruvian Bats with Comments on Natural History - University of Kansas Science Bulletin 49: p45-86.
Wied-Neuwied MP zu 1821 - Reise Nach Brasilien in de Jahren 1815 bis 1817 - Heinrich Ludwig Brönner, Frankfurt.
Wilkinson GS, Boughman JW 1998 - Social Calls Coordinate Foraging in Greater Spear-nosed Bats - Animal Behaviour 55: p337-350.
Willig MR 1985 - Reproductive Activity of Female Bats from Northeast Brazil - Bat Research News 26: p17-20.
Designed by Paul Smith 2006. This website is copyrighted by law.
Material contained herewith may not be used without the prior written permission of FAUNA Paraguay.
Photographs on this web-site are used with permission.


