

Designed by Paul Smith 2006. This website is copyrighted by law.
Material contained herewith may not be used without the prior written permission of FAUNA Paraguay.
Photographs on this web-site were taken by Paul Smith, Hemme Batjes, Regis Nossent, Frank Fragano,
Alberto Esquivel, Arne Lesterhuis, José Luis Cartes, Rebecca Zarza and Hugo del Castillo and are used with their permission.
Noctilio leporinus (Linnaeus 1758)
TAX: Class Mammalia; Subclass Theria; Infraclass Metatheria; Order Chiroptera; Suborder Microchiroptera; Superfamily Noctilionoidea; Family Noctilionidae (Hoofer et al 2003, López-González 2005, Myers et al 2006). The genus Noctilio, Linnaeus 1766, is the only genus in the family and contains two species, both of which are present in Paraguay. The origin of the name Noctilio is uncertain but is probably derived from the Latin “noctis” meaning night or perhaps from the French Noctilion meaning “bat” from the same root. The species name leporinus is Latin meaning “hare-like” presumably in reference to the hare-like cleft lip of the genus. No type specimen exists, the original description by Linnaeus being based on a text written by Seba (1734). The type locality was restricted to Surinam by O.Thomas (1911). The species name Noctilio labialis, long used to refer to Noctilio albiventris was demonstrated to be based on a misidentified specimen of this species (Hershkovtiz 1975, Davis 1976). The name Noctilio vittatus Schinz (1821) was stated by Cabrera (1938) to refer to the phenotype with a pale line along the back that had been described by Desmarest (1818) as Noctilio dorsatus and was not suggested as a replacement name for it. There are three recognized subspecies, populations in Paraguay being attributed to N.l.rufescens (Olfers 1818) (Type locality Paraguay based on a description of “chauve-souris rougeâtre” by de Azara 1801) by Davis (1973) who described it as the "largest and palest subspecies of the species". Myers & Wetzel (1983) concluded that a small sample from Paraguay averaged larger than examples of N.l.mastivus from the Amazon Basin, and López-González (2005) reached a similar conclusion based on a larger sample. Taddei et al (1986) state that individuals from southeastern Brazil are closer to populations from Bolivia and Argentina than Amazonian forms and suggest possible clinal variation in size decreasing towards the south-east and north-east in South America, but note that the data does not exist to affirm that theory. López-González (2005) concluded that a thorough review of the geographic variation of the species is required before the proposed subspecies can be considered validated. Lewis-Oritt et al (2001) estimated that based on molecular evidence this species derived from a N.albiventris-like ancestor 0.28-0.7 million years ago and that piscivory represents a derived morphologic state allowing the two species to exploit different feeding zones without the need for extensive divergence in physiologic or morphologic characters. Synonyms adapted from Hood & Knox Jones Jr (1984), López-González (2005) and Gardner (2007).
Vespertilio leporinus Linnaeus 1758:32. Type locality "America". Restricted to Surinam by O.Thomas (1911).
Vespertilio minor Fermin 1765:9. Name unavailable.
Noctilio americanus Linnaeus 1766:30. Junior objective synonym.
Pteropus leporinus Erxleben 1777:130. Name combination.
Vespertilio labialis Kerr 1792:93. Type localities "Peru and the Musquito Shore". Restricted to Lower Ucayali Region, Loreto, Peru by Hershkovitz (1949).
[Vespertilio] Mastivus Vahl 1797:132. Type locality "Insula St. Crucis Americae" (=St. Croix, US Virgin Islands).
Noctilio novemboracensis Lacépède 1799:16. No type locality.
Noctilio leporinus Illiger 1815:109. First use of current name.
[Noctilio] ?rufescens Illiger 1815:109. Nomen nudum.
N[octilio]. rufescens Olfers 1818:225. Type locality "Paraguay". Name based on de Azara (1801).
Noctilio unicolor Desmarest 1818:15. Type locality "l´Amerique Meridionale". Probably referring to Brazil (Hood & Knox Jones Jr 1984).
Noctilio dorsatus Desmarest 1818:15. Type locality "l´Amerique Meridionale"
Noctilio vittatus Schinz 1821:870. Type locality "Ostküste von Brasilien".
Celaemo brocksiana Leach 1821:70. No type locality.
Noctilio rufus Spix 1823:57. No type locality. Probably "Amazonian Brazil" (Hood & Knox Jones Jr 1984).
Noctilio rufipes d´Orbigny 1837: Plate 9, fig 4. Type locality "les grands foréts qui Bordent le Rio de San-Miguel au pays des Sauvages Guarayos (Bolivia)". Restricted to Rio San Miguel, Pampas de los Guarayos, Santa Cruz, Bolivia by Gardner (2007).
Noctilio macropus Pelzeln 1883:37. Nomen nudum taken from Natterer´s manuscripts.
Noctilio longipes Pelzeln 1883:37. Nomen nudum taken from Natterer´s manuscripts.
Noctilio intermedius Pelzeln 1883:37. Nomen nudum taken from Natterer´s manuscripts.
Noctilio leporinus mastivus True 1884:603. First use of current subspecific name.
Noctilio leporinus mexicanus Goldman 1915:316. Type locality "Papayo, Guerrero, Mexico".
Noctilio leporinus rufipes Cabrera 1938:14. Name combination.
Noctilio leporinus rufescens Hershkovitz 1959:340. First use of current subspecific name.
ENG: Greater Bulldog Bat (Hood & Knox Jones Jr 1984, Barquez, Giannini & Mares 1993), Fishing Bat (Hood & Knox Jones Jr 1984, Davis 1973, Eisenberg 1989), Mexican Bulldog Bat (Redford & Eisenberg 1992), Bulldog Fishing Bat (Parera 2002), Large Fishing Bat (Fischthal & Martin 1978), Hare-lipped Bat (Gudger 1945), Rabbit-nosed Night-flying Bat (Tomes 1860), Fish-eating Bat (Goodwin & Greenhall 1961), Surinam Fishing Bat (Goodwin & Greenhall 1961).
ESP: Murciélago pescador (Diaz & Barquez 2002), Murciélago pescador grande (Barquez, Giannini & Mares 1993), Murciélago buldog mayor (Emmons 1999), Pescador grande (Redford & Eisenberg 1992).
GUA: Mbopi pyta (Emmons 1999), Mbopi pyta guasu (FAUNA Paraguay 2006).
DES: This is an extremely large bat, with a protruding nose lacking a nose leaf, a strongly swollen and cleft forelip which exposes the large canines and pointed incisors. The chin is prominent and has conspicuous lateral ridges, and there are internal cheek pouches. The forward-leaning ears are separate, long, narrow and pointed with a lobed tragus. Ears are naked and brownish and furred only at the base. That tail is more than half the femoral length and extends to about one-third of the uropatagial length, the tip of the tail pointing free on the dorsal surface. Uropatagium extends beyond the extremely large and robust feet and there is a well-developed bony calcaneous. Pelage short. Paraguayan specimens are typically brownish dorsally, with some individuals more rufous-orange in colour (representing a larger proportion of the population than in Noctilio albiventris, but still a minority amongst all specimens). Davis (1973) suggested that the paler, more orange specimens may be the result of wear or bleaching or both. However Bordignon & França (2004) working with the species in southern Brazil noted that darker colouration was related to body mass and suggested that darkening of the pelage was related to sexual maturation - with males darkening from light yellow to brown with increased weight and females darkening from light yellow to greyish. However their sample size was small (n=23 males, n=16 females). Typically there is little difference in colour between the dorsum and the venter. A thin longitudinal line along the dorsal surface is usually paler than the rest of the pelage and may extend up between the ears. Wings long, narrow and pointed. Wing and tail membranes are brown and semitranslucent. Flanks naked. Live specimens have a strong musky odour. CR - Skull robust with high, deep braincase, flared mastoids and prominent sagittal crest (more so in males). Lacks well-developed post-orbital processes. Palate complete and closed anteriorly, appearing concave when viewed laterally but flat longitudinally. Premaxillaries fused to maxillaries and nasal and palatal branches fused together. Rostrum arched, about half the length of brain case and nares tubular and forward-opening. Maxillary toothrows approximately parallel. Auditory bullae small and covering half of the cochlea. (Hood & Knox Jones Jr 1984). López-González (2005) gave the following measurements for Paraguayan specimens (male n=43-45 female n=22-23): Greatest Skull Length male 24.4mm (+/- 0.46) female 25.9mm (+/- 0.74); Condylobasal Length male 23.5mm (+/- 0.44) female 24.8mm (+/- 0.36); Transverse Zygomatic Width male 18.9mm (+/- 0.32) female 20.1mm (+/- 0.32); Mastoid Width male 16.9mm (+/- 0.46) female 18.6mm (+/- 0.51); Interorbital Constriction male 6.8mm (+/- 0.14) female 7.1mm (+/- 0.18); Width Across Upper Molars male 12.2mm (+/- 0.21) female 12.8mm (+/- 0.33); Width Across Upper Canines male 8.4mm (+/- 0.22) female 9.2mm (+/- 0.26). DF: I2/1 C1/1 P 1/2 M 3/3 = 28. Upper incisors sharply-pointed and crowded around midline between canines. Innrmost pair of incisors twice as high as they are long and with shafts that curve outwards distally. Outer pair single-cusped, smaller and located slightly behind inner pair. Lower incisors equal and also crowded at the midline, with crowns longer than they are high and upper surface concave but not bilobed. Upper canines with distinct oblique cingulum, no secondary cusps and slightly concave with median ridge. Lower canines with slight twist in the shaft near the middle.Upper premolar twice as broad as it is long and prominently cusped. First and second upper molars inequal, prominently cusped and with strongly concave posterior surface so that they are separated by clear gaps. Crown area of M3 about half that of M2 but prominently cusped. (Hood & Knox Jones Jr 1984). López-González (2005) gave the following measurements for Paraguayan specimens (male n=45 female n=23): Upper Tooth Row male 9.9mm (+/- 0.19) female 10.4mm (+/- 0.24); Lower Tooth Row male 10.6mm (+/- 0.21) female 11.2mm (+/- 0.23). CN: 2n=34 FN= Originally published as 58 and later revised to 62. Graded series of 13 submetacentric and three pairs of acrocentric autosomes. X chromosome is a medium-sized metacentric and Y a small acrocentric chromosome. (Hood & Knox Jones Jr 1984).
MMT: One of the largest Paraguayan bats and the larger member of its family with wing length >2.5 times the head and body length. There is marked sexual dimorphism in size and in the development of the sagittal crest, both of which are larger in males than in females. López-González (2005) gave the following measurements for Paraguayan specimens (male n=43-45 female n=22-23): TL male 117.6mm (+/- 3.41), female 125.1mm (+/- 6.77); TA male 27mm (+/- 2.48), female 30.1mm (+/- 3.74); FT male 30.2mm (+/- 1.24), female 32.4mm (+/- 1.19); FA male 85.5mm (+/- 1.91), female 87.6mm (+/- 1.98); EA male 29.6mm (+/- 2), female 30.4mm (+/- 1.62); Length of Third Digit male 78.9mm (+/- 1.52), female 81.6mm (+/- 2.17) comprises c65% of wingspan; WT (male n=18 female n=8) male 53.5g (+/- 4.63), female 62.8g (+/- 7.6).
SSP: Bulldog bats can be immediately recognised on account of their large size, massive feet, pointed ears and "hare-lip". The two component species however are extremely similar to each other and can be distinguished with certainty only on the basis of size and body measurements. This is the larger of the two species with forearm length >80mm, hindfoot length>28mm and the combined length of the tibia ad hindfoot is greater than 80% of the forearm length. The confusion species Noctilio albiventris has a forearm length <70mm, hindfoot length <27mm, and the combined length of the tibia and hindfoot is less than 70% of the forearm length. Hood & Pitocchelli (1983) further state that this species generally has a wingspan c500mm and weight c50g compared to c400mm and c40g in Noctilio albiventris. When comparing skulls the condylobasal length is >21mm in this species and <21mm in albiventris. Though there may be some overlap between the largest albiventris and smallest leporinus in other skull measurements, Hood & Pitocchelli (1983) note that the length of the upper toothrow is rarely more than 8mm in albiventris and rarely less than 9mm in leporinus. The first and second molars of this species are clearly separated unlike those of albiventris.
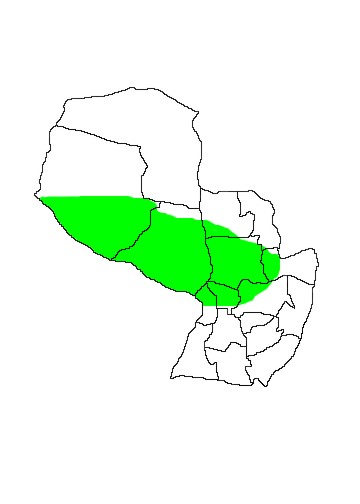
DIS: Widely distributed from western Sinaloa and eastern Veracruz in Mexico, through much of the Caribbean and south through South America to northern Argentina and southern Brazil. The distribution is apparently patchy and it is confined to major lowland river basins, seasonally-flooded and coastal areas and is absent from arid regions. Davis (1973) recognised three subspecies: N.l.mastivus (=N.l.mexicanus Goldman) in the northern part of the range through Central America and the Caribbean to northern and eastern Colombia, Venezuela and north-western Guyana; N.l.leporinus in the Amazon Basin of Brazil and Peru, the Guyanas and coastal Brazil as far south as Rio de Janeiro. The population in Bolivia, Paraguay, northern Argentina and southern Brazil was attributed to N.l.rufescens (=N.l.rufipes d´Orbigny) however see the taxonomic section for a discussion of the validity of these subspecies. In Paraguay this species has a more northerly distribution than N.l.albiventris being found in humid or seasonally-flooded areas of the southern Chaco departments of Boquerón and Presidente Hayes and across the northern Orient of Paraguay as far south as Departamento Cordillera.
HAB: Associated with temporary and permanent freshwater lagoons and slow-moving bodies of water of the Humid Chaco and its associated habitats. Its appearance in a given area is dependent on the presence of water and the species moves locally in times of drought. It is much less frequent in eastern Paraguay where it occurs in similar habitats, including suitable water courses in humid forest. In other areas of their range they may be found in coastal or tidal habitats. The species is relatively tolerant of human habitation and is found in urban areas where suitable hunting habitats are available nearby (Alava & Carvajal 2004).
MAP 23: Noctilio leporinus
ALI: This is one of the few bat species to have developed piscivory, aided by the extremely large hind feet, deeply-curved claws and scoop-like usage of the uropatagium. Bats are able to hunt only from a smooth water surface, reaching foot first and scooping with the huge feet and curved claws. The long claws are used to spear fish (Bloedel 1955) or to hook into the leg joints of large insects (Altenbach 1989), and a single foot may be used to catch smaller prey (Schnitzler et al 1994). Bats that approach the water face first are apparently drinking. Though Bloedel (1955) suspected that fishing trawls represented random drags over areas with high prey density, he noted that the bats attention was drawn by fish that broke the surface, and Suthers (1965) later demonstrated that they in fact can actively search for fish, detecting minute ripples of the water surface by echolocation. They are however incapable of locating submerged prey and bats may even attack the protruding dorsal fins of fish much larger than themselves apparently unaware that the bulk of the fish lies below the water surface (Schnitzler et al 1994). Schnitzler et al (1994) documented various search strategies in wild fishing bats which they defined as follows (see vocalisations section for echolocation differences associated with these strategies): 1) High Search Flight within 20-50cm of the water surface, in which a flying bat reacts to fishes that break the surface with pointed dips. During High Search Flight there are 4-5 wingbeats/s and flight speed is 6.6-7.4m/s. Detection distance of prey was estimated at 120-300cm using this method; 2) Low Search Flight within 4-10cm of the water surface with body parallel to the water, legs extended backwards and feet cocked and poised 2-4cm above the water surface. During Low Search Flight there are 5-6 wingbeats/s and flight speed is 5.6-6.6m/s. Makes rapid snappy dips at points where fish break the water surface. Detection distance of prey was estimated at 66-78cm using this method; 3) Directed Random Rake consistent with the observations of Bloedel (1955), this consists of long trawls of c10m with the claws breaking the water surface in areas of high prey activity; 4) Memory Directed Random Rake as for Directed Random Rake but performed in the absence of obvious prey activity in areas where the bat has previously had high success rates. Random Rake trawls were performed typically with the legs at a 120º angle, claws submerged for 1-2cm and calcaneum and uropatagium folded upwards away from contact with the water. During raking the wingbeat rate is reduced to 6-7 beat/s and flight speed to 5.2m/s. Under experimental conditions Altenbach (1989) noted that the claws are dipped into the water only a few centimetres prior to the prey and the feet are rarely submerged deeper than the two distal phalanges (consistent with Low and High Search Flight strategies). Bloedel (1955) however recorded trawls of up to 2m (consitent with Random Rake strategies). Suthers (1965) reported hyperextension of the feet when fishing, and though this was not photographed by Altenbach (1989) it was concluded that such hyperextension would be an adaptation for capture of larger, heavier prey. During level flight hunting bats hold the calcaneum posteromedially and the tail and uropatagium lie in a plane between the hind limbs. When approaching aquatic prey the tail is elevated, lifting the uropatagium and avoiding its contact with water. Wingbeats are co-ordinated with the moment of capture so that it coincides with a downstroke and caudal swing of the limbs, providing the force necessary to lift the prey item from the water. The food item is then brought forward towards the mouth and at the time of transfer the uropatagium is again extended to span the area between the feet. Transfer of prey to the mouth usually occurs within 2m of the capture point and takes c600ms (Altenbach 1989). Schnitzel et al (1994) noted that following a successful capture that the bats would spend 2 to 5 minutes consuming the fish on the wing and storing it in the cheek pouches. Wenstrup (1984) demonstrated that captive bats would respond to splashes on the water surface by immediately raking, even if no fish was present and Brooke (1994) noted similar responses to splashing schools of fish by bats in a wild setting in Puerto Rico. There is no evidence to suggest that vision or smell is employed in the detection of prey, but Schnitzler et al (1994) could not rule out the possibility that other non-echolocation clues were used in prey detection. The species shows certain specialisations in gastric morphology and histochemistry which are adaptations for a fish diet. The presence of cheek pouches was also previously considered an adaptation for fish-eating, but it is shared by the largely insectivorous Lesser Bulldog Bat which would seem to suggest that it evolved for other reasons. Fish however are not the only component of the diet and examined stomachs have been found to also contain insects and aquatic crustaceans. Brooke (1994) suggested that at least on Puerto Rico there was a seasonal variation in the diet of the species with insects (mostly beetles and moths) predominating in the wet season and fish becoming more prominent during the dry season. Both freshwater and pelagic fish were taken, with introduced Tilapia Oreochromis mossambicus predominating among the freshwater species. It was concluded that such fish were typically 34-57mm long and 5-6g in weight. The size range of marine fish that were taken was between 4-12cm and 3-12g in weight. Scorpions, crabs, shrimps and terrestrial insects were also recorded in the diet. The species was observed taking aerial insects away from water, around street lights, over fields and over roads. Insects were generally taken in flight whereas fish and terrestrial invertebrates were taken with the feet. Typically flying insects were captured not with the feet but net-like in the wing and tail membranes, and foraging flights lasted 46-87 minutes. Brooke (1994) concluded that as an aerial insectivore and piscivore the species has a flexible foraging strategy that enables it to adapt to changing local conditions. Interestingly Benedict (1926) describes bats of this species taking dead fish abandoned by pelicans on Trinidad during late afternoon, and adds that the stomachs of all the specimens collected contained "exclusively fish". Novick & Dale (1971) induced captive specimens to capture crickets that were thrown onto the water and also observed them take crickets that had crawled onto land, but they did not see the bats consume the insects. Goodwin (1928) had earlier concluded that insects were in fact the main element in the diet, a conclusion possibly explicable as a result of seasonal variation in prey choice documented by Brooke (1994). Insect prey in Central American stomachs includes Hymenoptera (winged ants Solenopsis), Orthoptera (Gryllotalpidae, Gryllidae), Coleoptera (Dytiscidae, Carabidae, Hydrophilidae, Cerambycidae, Scarabaeidae, Elateridae), Blattaria and Hemiptera (Hood & Knox Jones Jr 1984). Altenbach (1989) estimated capture success rates of 91% for stationary insects and 53% for dead fish 30-50mm long under experimental conditions, whilst Schnitzler et al (1994) estimated success rates in wild bats at one fish per 50-200 "rakes" or 0.5-2% with "near captures" at about 2-3 times that rate. Bloedel (1955) describes captive individuals presented with dead fish as "crawling about on the floor until they blundered into a fish", seizing the fish with the teeth and climbing to top of the cage to consume it. He estimated that his bats consumed 30 to 40 small fish each night, with a maximum of 38 recorded for one of his captive specimens, but added that wild bats probably consumed less owing to the lower density of fish in wild situations and the addition of insect matter in the diet. Goodwin (1928) makes reference to an apparently upublished description by JH Gosse in Jamaica who fed captive specimens on cockroaches. The consumption of the animals was accompanied by "a loud cranching (sic) of the teeth" not attributable to the horny parts of the insect as it was also noted when consuming soft-bodied items. Mastication was rapid but also a long process and "performed almost exclusively by the canines". Upon mastication the pieces were allowed to fall into the cheek pouches, with one pouch being almost filled before transfer to the other pouch began. Once the cockroach had been completely masticated a contorsion of the jaw muscles returned the food to the mouth where it was again masticated before swallowing, a process likened to "rumination". The same individual was offered small pieces of bird flesh which it "ruminated" in much the same way, but eventually expelled without being swallowed. Observations on wild bats in Costa Rica noted that for the most part bats foraged alone and that males at least may maintain foraging territories, breaking off their hunting to antagonistically fly at other individuals that encroached. Individual bats foraged in wide loops or figure of eight patterns, retracing the same patterns continuously and flapping their wings furiously when turning, often resulting in the animal gaining height momentarily. (Schnitzler et al 1994). A specimen captured in a mist net in the Paraguayan Chaco released a partly eaten frog (Myers & Wetzel 1983).
REP: Well-studied in Central America but little data available for the South American range. Females are monovular and give birth to a single young once a year. In Central America gestation begins during the winter with parturition during the late spring and early summer. A lactating female with young was found in Paraguay during April (autumn) and in Argentina there is apparently no evidence of birthing in spring and early summer (Parera 2002). Hooper & Brown (1968) concluded that this species breeds earlier in the year than the Lesser Bulldog Bat in Costa Rica, suggesting this as a further mechanism allowing sympatry of the two species. Birthing is apparently highly-synchronised. There is some evidence of a second reproductive peak in the breeding cycle in late summer and autumn at least in some parts of the Central American range, data to confirm this conclusively has not yet been forthcoming.(Hood & Knox Jones Jr 1984). Wehekind (1956) documents a female giving birth to a juvenile bat whilst hanging upside-down. Silva Taboada (1979) noticed that juveniles did not leave the colony until almost 1 month old when they had reached almost adult size and that adult males and females remained at the roost during the time that juveniles were present - suggestive of a high degree of brood care. A pocket-like fold of skin around the scrotum of males produces a strong musky odour which may be related to the reproductive cycle.
BEH: Activity Levels Activity patterns differ from Noctilio albiventris and the species shows no peaks of activity, being active throughout the night (Hooper & Brown 1968). Activity levels vary day to day presumably being affected by various factors such as food availability, wind strength and/or moon cycles (Schnitzel et al 1994). In Cuba the bats were observed to leave their roosts a few hours earlier during the coldest months of the year (Silva Taboada 1979) and in Costa Rica bats leaving the roost performed long "passing" flights appearing from one direction and disappearing into the distance at a height of c0.5-1m above the water surface without attempting any captures (Schnitzler et al 1994). Locomotion Despite the long wings and high aspect ratio, this species flies with slow, deliberate wing beats. They are apparently able to swim when necessary and Goodwin (1928) describes bats knocked into water as able to swim "with considerable ease" despite injuries inflicted by the attempts to capture them, using "both wings under the water as oars" and unlike other bats "with only its head above the water surface". Most bats knocked into the water by Goodwin (1928) swam directly for the shore and hid in cover, but others swam "three or four times round the pond in a vain attempt to escape". The somewhat gruesome account of the attempt to procure specimens continues by noting that bats knocked into water but not hard enough to break bones were able to rise from the water surface and fly away, whilst specimens held under water for a few minutes "soon drowned". Roosts Roosts are often large with up to 75 individuals reported in Trinidad (Goodwin & Greenhall 1961), 30-150 in Argentina (Parera 2002) and may sometimes contain several hundred individuals. Colonies have a strong musky odour possibly due to the production of strong-smelling secretions produced by a fold of skin around the scrotum of males, and Goodwin (1928) suggested that the odour was strong enough from passing bats to be able to identify them away from the colony by smell alone. He notes that whilst the smell of bats taken in May from Botany Bay, Trinidad was strong it was not objectionable, yet specimens from Porto Rico (sic) taken in February had an offensive smell that seemed to "cling to my hands for days". Though often quoted as preferring rocky or cave roosts this would seem to be more the case in coastal areas and the species has been known to roost in hollow trees over much of its Central American range, likely doing so also in Paraguay where there is a shortage of rocky areas (Hood & Knox Jones Jr 1984). Roosts may contain males, females and juveniles together, but at least one roost in Honduras contained 16 males (Carter et al 1966) and another roost in Guatemala had a predominance of immature individuals (Dickerman et al 1981). Goodwin & Greenhall (1961) suggested that at least on Trinidad the sexes may be segregated during parturition but roosted together for the rest of the year. Parasites Almost all available data is from Central America. Ticks, mites, batbugs, batflies, nematodes and trematodes have been recorded. Spinturnicid mites Periglischrus aitkeni in Panama, P.ojastii in Venezuela; Labiocarpid mites Parakosa maxima and P.tadarida in Venezuela, Notoedres noctiliones in Cuba; Macronyssid mite Chiroptonyssus venezolanus in Cuba, though the species is more often associated with the Molossidae; Argasid mite Ornithodoros dusbabeki in Cuba, O.hasei in Panama, O.boliviensis and O.tiptoni in Venezuela; Sarcoptid mite Teinocopterus sp in Trinidad; Batbugs Cimicidae Latrocimex sp in Trinidad; Trematode Pygidiopsis macrostomum in Cuba, Postorchigenes paraguayensis in Paraguay (Fischthal & Martin 1978); Nematodes Contracaecum sp., Tricholeiperia proencai, Spirocera lupi, Capillaria viguerasi in Cuba; Batflies Streblidae Noctiliostrebla aitkeni, N.dubia, N.megastimata, N.traubi, Paradyschiria fusca, P.lineata and Xenotrichobius noctilionis. These three genera are frequently associated with Noctilio and perhaps even confined to the family. The presence of the Polyctenid Hemipteran Hesperoctenes fumarius was considered to be accidental. Presley (2005) found 553 parasites on 28 specimens of this bat in Paraguay, the assemblage being dominated by three monoxenous streblids (Noctiliostrebla aitkeni, N.dubia, and Paradyschiria fusca) and O. hasei. In addition, an apparently undescribed macronyssid (Steatonyssus sp.) occurred regularly on N. leporinus.
VOC: Constant frequency and frequency modulated portions of the echolocation of this species are adapted to long range sonar to improve their fish-catching ability, but are otherwise typical of other microchiropterans (Hood & Knox Jones Jr 1984). Two distinct echolocation pulses were detected by Suthers (1965). The first included constant frequency pulses beginning around 60kHz that did not dip below 50kHz. A second pulse type also began at 60kHz but was modulated downwards in frequency by more than an octave. Both pulses had a duration of c8ms for captive bats, with up to 14ms recorded in wild specimens. When pursuing prey repetition rate rises from 16 to 200/s and the constant frequency pulses are all modulated. Pulse duration declines to c1/s in the moment just prior to prey capture. Schnitzler et al (1994) documented the variation in echolocation pulses as they corresponded to differing foraging strategies defined in the alimentation section above. 1) During the High Search Flight it emits groups of 2-4 signals containing at least one constant frequency pulse and one mixed constant/frequency-modulated pulse. Constant frequency pulses averaged 13.3ms in duration up to maximum of 17ms, and had a frequency of 52.8-56.2kHz. The constant frequency component of the mixed pulse averaged 8.9ms with the modulated section 3.9ms. Frequency modulated components have an average bandwidth of 25.9kHz. 2) During the Low Search Flight it emits long series of short mixed constant/frequency-modulated pulses with an average duration of 5.6ms, 3.1ms referring to the constant frequency part and 2.6ms to the frequently-modulated section. Average pulse interval was 20ms indicating searches for short range targets. Goodwin (1928) noted a "sharp crunching sound as if grinding the horny parts of a large beetle between its teeth" as being made by individuals approaching water, but never by individuals leaving water.
HUM: This is a large and conspicuous bat that attracts the attention of human populations wherever it occurs. Local belief, for a long time accepted by the scientific community, that the uropatagium is used as a sort of scoop when capturing fish has since been proved to be unfounded, but reflects the difficulty in observing the precise fishing technique accurately under wild conditions.
CON: Globally considered to be of Low Risk Least Concern by the IUCN, click here to see the latest assessment of the species. Considered potentially vulnerable in Paraguay because of its specific habitat requirements, but common in areas of suitable habitat in the Humid Chaco. Much less numerous in eastern Paraguay were suitable habitat is thin on the ground and considered rare in humid forest areas. (López-González 2005). Currently the Humid Chaco is relatively well-protected, but increasing land pressure in eastern Paraguay and the expanse of the agricultural frontier has led to the draining of many wetland areas. Furthermore a lack of available land for farming in eastern Paraguay, coupled with the increased accessibility of the Chaco with the paving of the Ruta Trans-Chaco has caused an increase in the number of people interested in settling what were previously considered undesirable areas in the Chaco. In the Humid Chaco conversion of palm savanna to ranchland or drainage for agriculture could present potential threats to the species and clearing and burning of such areas to create pasture land may inadvertently eliminate suitable roosting sites. The species is sensitive to chemical and heavy metal pollution and run-off (Parrera 2002).
Citable Reference: Smith P (2008) FAUNA Paraguay Online Handbook of Paraguayan Fauna Mammal Species Account 23 Noctilio leporinus.
Last Updated: 25 February 2009.
References:
Alava JJ, Carvajal R 2004 - Ocurencia de Noctilio leporinus (Chiroptera: Noctilionidae) en la Zona Urbana y Alrededores de Guayaquíl, Ecuador - Chiroptera Neotropical 10: p183-187.
Altenbach JS 1989 - Prey Capture by the Fishing Bats Noctilio leporinus and Myotis vivesi - Journal of Mammalogy 70: p421-424.
Azara F de 1801 - Essais sur l´Histoire Naturelle des Quadrupèdes de la Province du Paraguay - Charles Pougens, Paris.
Barquez RM, Giannini NP, Mares MA 1993 - Guide to the Bats of Argentina - Oklahoma Museum of Natural History, Norman, Oklahoma.
Benedict JE 1926 - Notes on the Feeding Habits of Noctilio - Journal of Mammalogy 7: p58-59.
Bordignon MO, Oliveira França A de 2004 - Variacões na Coloração da Pelagem do Morcego-pescador Noctilio leporinus (L. 1758) (Mammalia: Chiroptera) - Revista Brasileira de Zoociencias 6: p181-189.
Bloedel P 1955 - Hunting Methods of Fish-eating Bats, particularly Noctilio leporinus - Journal of Mammalogy 36: p390-399.
Brooke AP 1994 - Diet of the Fishing Bat Noctilio leporinus (Chiroptera: Noctilionidae) - Journal of Mammalogy 75: p212-218.
Cabrera A 1938 - Dos Nuevos Murciélagos para la Argentina - Notas Zoologicas Museo de la Plata 3: p5-14.
Carter DC, Pine RH, Davis WB 1966 - Notes on Middle American Bats - Southwestern Naturalist 11: p488-499.
Davis WB 1973 - Geographic Variation in the Fishing Bat Noctilio leporinus - Journal of Mammalogy 54: p862-874.
Davis WB 1976 - Geographic Variation in the Lesser Noctilio Noctilio albiventris - Journal of Mammalogy 57: p687-707.
Desmarest A 1818 - Noctilion ou Bec de Lièvre - Nouveau Dictionnaire de Histoire Naturelle 23: p14-16.
Díaz MM, Barquez RM 2002 - Los Mamíferos de Jujuy, Argentina - LOLA, Buenos Aires.
Dickerman RW, Koopman KF, Seymour C 1981 - Notes on the Bats of the Pacific Lowlands of Guatemala - Journal of Mammalogy 62: p406-411.
Eisenberg JF 1989 - Mammals of the Neotropics: Volume 1 The Northern Neotropics - University of Chicago Press, Chicago.
Eisenberg JF, Redford KH 1999 - Mammals of the Neotropics: Volume 3 The Central Neotropics - University of Chicago Press, Chicago.
Emmons LH 1999 - Mamíferos de los Bosques Húmedos de América Tropical - Editorial FAN, Santa Cruz.
Erxleben JCP 1777 - Systema Regni Animalis per Classes, Ordines, Genera, Species, Varietates cun Synonimia et Historial Animalium: Classis 1 Mammalia - Weygandianus, Leipzig.
Fermin P 1765 - Histoire Naturelle de la Hollande Equinoxiale - Amsterdam.
Fischthal JH, Martin RL 1978 - Postorchigenes paraguayensis sp. n. (Trematoda: Pleurogenidae) A Digenetic Trematode from a Large Fishing Bat Noctilio leporinus rufescens Olfers from Paraguay - Acta Parasitologica Polonica 25: p217-221.
Gardner AL 2007 - Mammals of South America Volume 1: Marsupials, Xenarthrans, Shrews and Bats - University of Chicago Press.
Goldman EA 1915 - Five New Mammals from Mexico and Arizona - Proceedings of the Biological Society of Washington 28: p133-138.
Goodwin GG 1928 - Observations on Noctilio - Journal of Mammalogy 9: p104-113.
Goodwin GG, Greenhall AM 1961 - A Review of the Bats of Trinidad and Tobago - Bulletin AMNH 122: p187-301.
Gray JE 1827 - Synopsis of the Species of the Class Mammalia p 1-296: in The Animal Kingdom Arranged in Conformity with its Organization, by the Baron Cuvier with Additional Descriptions of all the Species Hitherto Named and Many Not Before Noticed by Edward Griffith and Others - GB Whittaker, London.
Gudger EW 1945 - Fisherman Bats of the Caribbean Region - Journal of Mammalogy 26: p1-15.
Hershkovitz P 1949 - Mammals of Northern Colombia, Preliminary Report Number 5: Bats (Chiroptera) - Proceedings of US National Museum 99: p429-454.
Hershkovitz P 1959 - Nomenclature and Taxonomy of the Neotropical Mammals Described by Olfers 1818 - Journal of Mammalogy 40: p337-353.
Hershkovitz P 1975 - The Scientific Name of the Lesser Noctilio (Chiroptera) with Notes on the Chauve-souris de la Vallee D´Ylo (Peru) - Journal of Mammalogy 56: p242-247.
Hood CS, Knox Jones Jr J 1984 - Noctilio leporinus - Mammalian Species 216.
Hood CS, Pitocchelli J 1983 - Noctilio albiventris - Mammalian Species 197.
Hoofer SR, Reeder SA, Hansen EW, Van den Bussche RA 2003 - Molecular Phylogenetics and Taxonomic Review of Noctilionid and Vespertilionid Bats (Chiroptera: Yangochrioptera) - Journal of Mammalogy 84: p809-821.
Hooper ET, Brown JH 1968 - Foraging and Breeding in Two Sympatric Species of Neotropical Bats, Genus Noctilio - Journal of Mammalogy 49: p310-312.
Illiger JKW 1815 - Ueberblick der Säugthiere nach Ihrer Vertheilung Über die Welttheile - Abhandl. König. Akad. Wiss. Berlin 1804-18811: p39-159.
Kerr R 1792 - The Animal Kingdom or Zoological System of the Celebrated Sir Charles Linnaeus: Class 1, Mammalia: Containing a Complete Systematic Description, Arrangement and Nomenclature of all the Known Species and Varieties of Mammalia - J Murray & R Foulder, London.
Lacépède BGE de la V 1799 - Tableau des Divisions, Sous-divisions, Ordres et Genres des Mammifères. Supplement to Discours d´Ouverture et de Clôture du Cours d´Histoire Naturelle Donné dans le Muséum National d´Histoire Naturelle, l´an VII de la République, et Tableau Méthodiques des Mammifères et de Oiseaux - Plassan, Paris.
Leach WE 1821 - The Characters of Three New Genera of Bats without Foliaceous Appendages on the Nose - Transactions of the Linnean Society of London 13: p69-72.
Lewis-Oritt N, Van den Bussche RA, Baker RJ 2001 - Molecular Evidence for Evolution of Piscivory in Noctilio (Chiroptera: Noctilionidae) - Journal of Mammalogy 82: p748-759.
Linnaeus C 1758 - Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, Cum Characteribus, Diferentiis, Synonymis, Locis 10th Ed. - Holmiae, L.Salvii.
Linnaeus C 1766 - Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, Cum Characteribus, Diferentiis, Synonymis, Locis 12th Ed. - Holmiae, L.Salvii.
López-González C 2005 - Murciélagos del Paraguay - Biosfera Numero 9.
Myers P, Espinosa R, Parr CS, Jones T, Hammond GS, Dewey A 2006 - The Animal Diversity Web (online). Accessed December 2007.
Myers P, Wetzel RM 1983 - Systematics and Zoogeography of the Bats of the Chaco Boreal - Miscellaneous Publications of the Museum of Zoology, University of Michigan 165.
Novick A, Dale BA 1971 - Foraging Behaviour in Fishing Bats and their Insectivorous Relatives - Journal of Mammalogy 52: p817-818.
Novak RM 1991 - Walker´s Mammals of the World 5th Ed Volume 1 - Johns Hopkins, Baltimore.
Olfers I 1818 - Bemerkubgen zu Iliger´s Ueberblick der S?ugethiere nach ihrer Vertheilung uber die Welttheile, Rücksichtlich der Südamerikanschen Arten (species). Abhandlung 10, Wilhelm Ludwig Eschwege´s Journal von Brasilien Vol 15, heft 2:p192-237 - Nueue Bibliothek des Wichtigsten Reisenbeschreibungen zur Erweiterung der Erdund Volkerkunde, FT Bertuch, Weimar.
d´Orbigny AD 1835 - Voyage dans l´Amerique Méridionale (le Bresil, la République Oriental de Uruguay, la République Argentine, la Patagonie, la République du Chile, la République de Bolivia, la République du Perou) Executé Pendant les Années 1826, 1827, 1828, 1829, 1830, 1831, 1832, 1833 - Pitois-Levrault et cie, Paris, Strasbourg.
Parera A 2002 - Los Mamíferos de la Argentina y la Región Austral de Sudamérica - Editorial El Ateneo, Buenos Aires.
Pelzeln A von 1883 - Brasilische Säugethiere. Resultate von Johan Natterer´s Reisen in den Jahren 1817 bis 1835 - Verbhandl. Kaiserl.-König. Zool.-bot Gesellsch, Wien 33 (supp).
Presley SJ 2005 - Ectoparasitic Assemblages of Paraguayan Bats: Ecological and Evolutionary Perspectives - Texas Tech University PhD Dissertation.
Redford KH, Eisenberg JF 1992 - Mammals of the Neotropics: Volume 2 The Southern Cone - University of Chicago Press, Chicago.
Seba A 1734 - Locupletissimi Rerum Naturalium Thesauri Accurata Descriptio, et Iconibus Artificiosissimis Expretio per Universam Physices Historiam - Amsterdam.
Schinz HR 1821 - Das Tierreich Eingetheilt nach dem Bau der Thiere als Grundlage ihrer Naturgeschichte und der Vergleichenden Anotomie von dem Herrn Ritter von Cuvier Vol 1 Säugethiere und Vögel - Stuttgart & Tübingen.
Schnitzler HU, Kalko EKV, Kaipf I, Grinnell AD 1994 - Fishing and Ecolocation Behaviour of the Greater Bulldog Bat Noctilio leporinus in the Field - Behavioural Ecology and Sociobiology 35: p327-345.
Spix JB von 1823 - Simiarum et Vespertilionum Brasiliensum Species Novae: Ou Histoire Naturelle des Esepecies Nouvelles de Singes et Chauves-souris Observées et Recueilles Pendant le Voyage dans L´interieur de Bresil - Typis Francisi Serephici Hübschmanni, Monaco.
Suthers RA 1965 - Acoustic Orientation by Fish-catching Bats - Journal of Experimental Zoology 158: p319-348.
Silva Taboada G 1979 - Los Murciélagos de Cuba - Cuba, Havanna.
Taddei VA, de Seixas RB, Dias AL 1986 - Noctilionidae (Mammalia, Chiroptera) do Sudeste Brasileiro - Ciencia e Cultura 38: p904-916.
Thomas O 1911 - The Mammals of the Tenth Edition of Linnaeus: An Attempt to Fix the Types of the Genera and the Exact Bases and Localities of the Species - Proceedings of the Zoological Society of London 1911: p120-158.
Tomes RF 1860 - Notes on a Third Collection of Mammalia made by Mr Fraser in the Republic of Ecuador - Proceedings of Zoological Society of London.
True FW 1884 - A Provisional List of the Mammals of North and Central America and the West Indian Islands - Proceedings of US National Museum 7: p587-611.
Vahl M 1797 - Beskrivelse paa tre nye Arter Flagermuse - Skrivt. Naturhist.-Selskavet Kjobenhavn 4: p121-138.
Wehekind L 1956 - Notes on some Trinidad Bats - Journal of Trinidad Field Naturalists Club p18-21.
Wenstrup JJ 1984 - Auditory Sensitivity in the Fish-catching Bat Noctilio leporinus - Journal of Comparative Physiology 155: p91-101.


