

A PRELIMINARY REVIEW OF THE
ECOLOGY OF THE ANURANS OF SAN RAFAEL NATIONAL PARK
David Gill
Pro Cosara/ECOSARA, Estación Ecologica de San Rafael, Alto Verá, Departamento Itapúa
Designed by Paul Smith 2006. This website is copyrighted by law.
Material contained herewith may not be used without the prior written permission of FAUNA Paraguay.
Photographs on this page were provided by David Gill and are used with their permission.
INTRODUCTION
The ecological importance of anurans and the possible threats they face
Researching the ecology of anurans has far-reaching consequences for both conservation and evaluation of environmental health. As amphibians, anurans have a permeable skin making them highly sensitive to both aquatic and terrestrial pollutants, thus making them excellent indicators of the health of an ecosystem (Barinaga, 1990). They are key members of tropical ecosystems where, not only do they often make up the highest vertebrate biomass (Beebee 1996, cited by Gardener et al. 2001), but also act as both predator and prey species, playing an important role in controlling arthropod abundance as well as providing a substantial prey base for other predators such as mammals, birds, reptiles and arachnids (Guyer 1990, cited by Gardener et al. 2001). Bearing this in mind it is disturbing that declines in anuran populations are increasingly being reported at a global scale. To cite a classic example, the population of the formerly abundant Golden Toad (Bufo periglenes) fell by 99% in a single year (1987) for reasons that at the time were unknown (Pounds and Crump, 1994). Since then, the documentation of anuran declines has accelerated at a worrying rate, particularly within specific and threatened eco-regions such as the Central Valley of California (Fisher and Schaffer, 1996), and the montane forests of Eastern Australia (Laurence, et. al, 1996). Frequently the causes of such sudden and catastophic declines are difficult to identify, as anuran populations may fluctuate naturally from year to year (Marsh and Trenham 2000) and long term studies are required to track changes in amphibian populations and to identify the causes behind them . Possible agents of decline are multiple and can include one or a combination of increased exposure to UV-B radiation, atmospheric pollutants, disease, climatic changes, exotic introductions and habitat destruction (Gardener et al. 2001). If, as recent evidence appears to suggest, anurans and other amphibians are severely threatened at a global scale, then it is imperative that the status of amphibian populations be closely monitored, especially within severely threatened habitats such as the Atlantic Forest of South America.
The status of the Atlantic Forest and its anuran fauna
Located in southeastern Brazil, eastern Paraguay, and the northern tip of Argentina, less than 8% of the original cover of the Atlantic remains, a situation is so critical that the habitat is listed as one of the five top hotspots for conservation priority in the world (Myer et al 2000). The impact of such severe deforestation on the anuran fauna are currently unclear due to absence of data about the distribution and population dynamics of Atlantic Forest species (Eterovick et al. 2005). What little data that is available has suggested that severe declines of at least some species are a reality, including the local extinction of Fritzina abausi from Boracéia, a segment of forest in southeast Brazil (Heyer et al. 1988). In a recent review, Eterovick et al. (2005) proposed that at least 18 species of anuran found in the Brazilian Atlantic Forest are sufficiently threatened to warrant immediate research and conservation attention. No such data is available for Paraguayan anurans, though the situation is likely to be equally as critical. This paper represents the first contribution towards the subject carried out in the San Rafael National Park, Departamento Itapúa in southern Paraguay.
The status of San Rafael National and its anuran fauna
San Rafael National Park (SRNP), the largest remaining segment of Atlantic Forest found within Paraguay. SRNP is mainly constituted of Upper Paraná Lowland Atlantic Forest, the most threatened type of habitat within the Atlantic Forest biome (Fragano and Clay, 2003). Though it is officially protected with the title of "Reserve Designated for a National Park" on the ground protection has been ineffective and the area remains under serious threat. Currently, despite the designation of the reserve as a "national park", all the land within the reserve is in private hands and the future of the area from a conservation perspective remains in doubt. Between 1989-2002 an average of 9.8km² of forest per year were removed from within the area and at present more than 22% of the park has been modified or removed (A. Parra et al. unpublished report, Esquivel et al. 2007).
The anuran fauna within San Rafael National Park (SRNP) was first evaluated during a brief "Rapid Ecological Assessment" in 1989 by the Museo Nacional de Historia Natural del Paraguay (MNHNP). Since then, colelcting expeditions, by the University of Carnegie (1995) and the University of Kansas (1996 and 2000) have added to the species list and Motte and Nuñez (2002), documented a total of 33 species of anurans in SRNP. To date no ecological study on the amphibians of SRNP has yet been published and this paper represents the first attempt to contribute such data.
AIMS AND OBJECTIVES
·To locate breeding sites and identify which species of anurans are breeding within them
·To document how the presence of breeding species varies across different microhabitats
·To document how the presence of breeding species varies across differing time zones
·To analyse how the presence of breeding species relates to temperature
·To document which species, though not shown to be breeding, are present within the study area
THE STUDY AREA
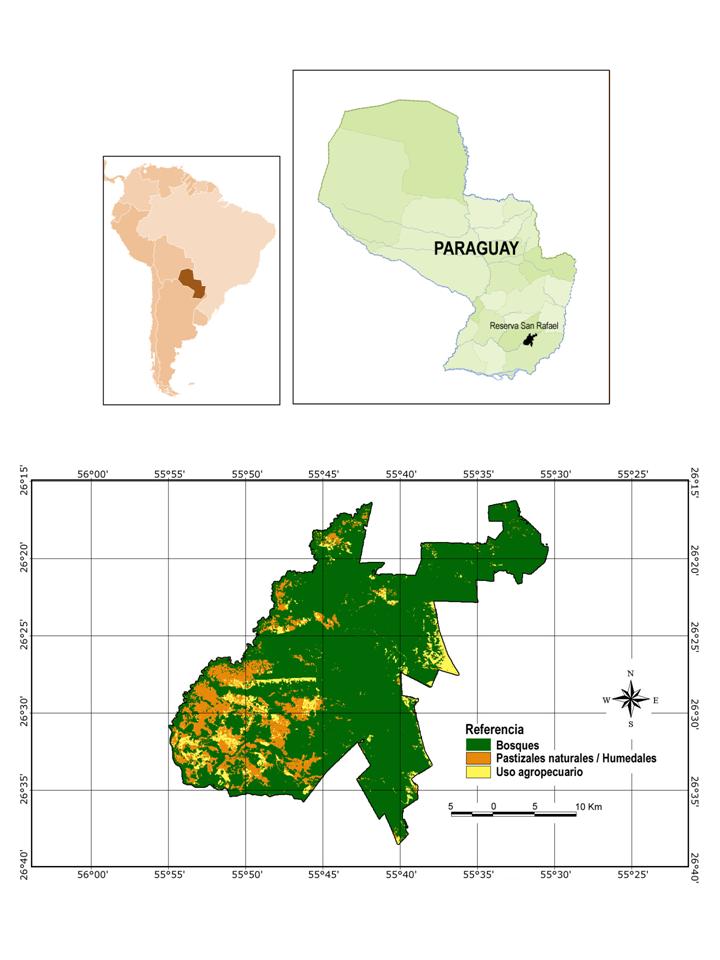
San Rafael National Park (SRNP) is located in the south of Paraguay above the Tebicuary river and inside the departments of Itapúa and Caazapa, see FIG 1. In total the park covers 748 km², including over 700km² of Upper Paraná Lowland Atlantic forest as well as areas of Mesopotamian Grasslands. Fieldwork was carried out at ECOSARA (Estación Ecológica San Rafael) based at Estancia Nueva Gambach PROCOSARA (26º38´S, 55º40´W) at the southern tip of SRNP. The property covers an area of 2.5km² and contains areas of both altered and original forest. In the centre of the area lies an artificial lagoon which is fed from the north by forest streams, and in turn feeds a network of small ponds and streams in meadows that surrounds the lagoon’s southern and western edges, see FIG 2.
FIG 1: Location of San Rafael National Park in south-east Paraguay. Vegetation cover is divided into three main classes - forest, grassland/wetland and anthropogenic areas. Map generated by Alberto Esquivel. (Click to enlarge)
Vegetation Structure (see FIG 2)
Forest
Descriptions of vegetation are taken from Esquivel et al., (2007). The property contains various types of forest habitat. Areas of primary forest (defined as areas with closed canopy with trees of a height greater than 20m and open understorey) still exist within the property. A localised hurricane in 2002 created large areas of regrowth forest - known locally as capueira - (open canopy with a trees of a height between 15-19m with a thick understorey). Common canopy species include Nectandra megapotamica, Chrysophyllum gonocarpum, Cabralea canjerana, Ficus enormis, Balfourodendron riedelianum and Bastardiopsis densiflora. Common understorey species include Ctinostemon concolor, Sorocea bonplandii, Piper sp., Inga marginata and Trichilia catigua. Lianas (Bignoniaceae, Apocynaceae, Dilleniaceae), epiphytes (Orchidaceae, Bromeliaceae, Araceae) and ferns (Pteridophyta) are also abundant. The area also contains segments of gallery forest, comprised of thick bamboos (Guadua sp.), that surround the streams entering the lagoon from the forest.
Meadows
These meadows are largely comprised of exotic short grass which are used as pasture for livestock. A variety of reed species and long grasses were found by the lagoon and other small ponds. Common example species include Typha sp.
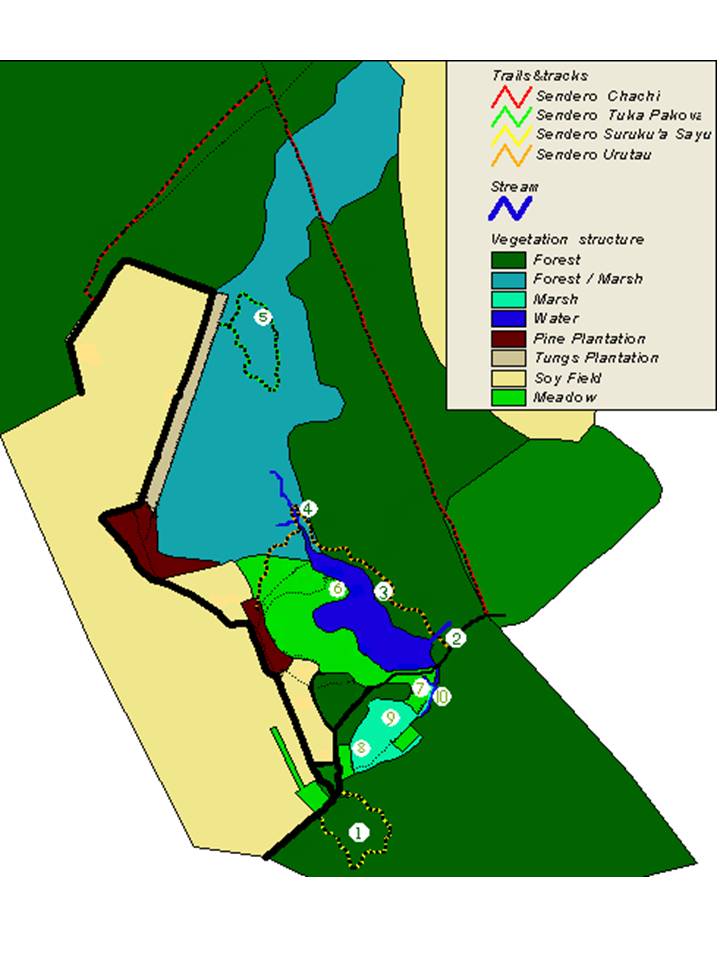
FIG 2: Vegetation map of the study site Estancia Nueva Gambach. Numbers 1 to 10 refer to study sites listed on Table 1. Map generated by Bernard Oosterbaan. (Click to enlarge)
METHOD
Moitoring anurans through calling behavior
As a result of the high predation risk, many anurans employ cryptic colouration or attempt to conceal themselves amongst vegetation or within leaf litter. (Alford and Richards, 1999). This creates certain difficulties when using random search tecnhiques to find anurans, and a variety of other systems must be employed to increase success rate. However, nearly all anuran species can be identified by their distinct calls. Males from each species are capable of producing a varied repertoire of calls, depending on the behavioral context (Toledo and Haddad 2005), but the type of call most widely recorded is the advertisement call, which is used to attract females and signify the occupation of a territory to rival males (Luddeke, 2001). Thus, the activity of anurans is most efficiently recorded during the breeding season, especially when, as is the case for most species, individuals aggregate en masse at particular sites (Beebee 1996, cited by Gardener et al., 2001). As a result, many studies assessing the size of anuran populations or communities concentrate sampling efforts around known breeding ponds (Marsh and Trenham, 2001).
Recording of vocalisations
Recordings were taken between the 23rd of March and the 16th of May, from a total of ten sites; five within the forest and five within the meadows (see Table 1). Sampling of vocalizations took place throughout the night (between 1800 and 0400), and in order to measure nocturnal variation each of the ten sites were sampled across five different time segments - 1800-2000, 2000-2200, 2200-0000, 0000-0200 and 0200-0400. Each site was sampled three times for each time segment, giving a total of 150 samples. Within each site the presence of all anuran species was documented, by recording vocalizations while actively searching a 30m transect. Only recordings localized within an estimated fifteen metres either side of this line were analysed and thus each site had an estimated area of 900m². Transect time was limited to 20 minutes. All vocalization samples were recorded using a Sennheiser microphone and Sony Hi-MD mini-disc player. Recordings were identified either through direct observation of a calling species, or through later analysis using the program Cool-edit Pro. Uploaded samples were then compared to other recordings in the ECOSARA Biodiversity Database (P.Smith unpub.) or published recordings (Stranek, 1995).
Forest Sites Vegetation Water Source
1. Surukua Original forest None
2. Urutau 1 Gallery forest Stream
3. Urutau 2 Degraded forest Large lagoon
4. Urutau 3 Degraded forest Ponds
5. Tuca Pacova 1 Original forest/marsh Ponds/streams
Meadow Sites Vegetation Water Source
6. Large lagoon Short grass and reeds Large lagoon and ponds
7. Small lagoon Reeds Small lagoon
8. Meadows 1 Reeds and tree cover Ponds
9. Meadows 2 Reeds Ponds
10. Meadows 3 Short grass and reeds Stream
Table 1 - Summary of vegetation and description of nearby water source for all ten study sites
Pitfall traps and personal observations
A total of 27 pitfall traps were active during the period 21 February to 18 May. Data was collected from four sites (see Table 2) all located within the forest. Pitfall traps were not located in marsh or meadow habits because frequent flooding rendered the results unusable. Each site consisted of nine buckets (35-38cm in diameter, and 40-45cm in depth), which were arranged 15 m apart from each other in a 3x3m grid, thus encompassing an area of 900 m². Traps were checked every morning and additonally all anuran sightings made during the study were dated and documented
Location Habitat Type Duration
Surukua Forest February 21st - May 18th
Chachi Original Forest February 21st - March 7th
Urutau Streams Gallery Forest March 11th - May 18th
Urutau Lagoon Degraded Forest April 22nd - May 18th
Table 2 - Dates that pitfall traps were operational.
RESULTS
Identity of breeding species, breeding sites and interspecific variation in habitat use
A total of 101 cuts of vocalisations were recorded with one cut representing the confirmation of presence of a given species in a given site at a given time. From these a total of seven species were identified: Renala ornata and R. schneideri (Familia Bufonidae), Odonotophrynus americanus (Familia Cicloramphidae), Physalaemus albonotatus (Familia Leiuperidae), Hypsiboas albopunctatus, H. caingua, and Scinax berthae (Familia Hylidae).
Site Species diversity Activity
Tuca Pacova 4 22
Meadows 2 3 18
Artificial Lagoon 1 2 20
Artificial Lagoon 2 2 14
Meadows 1 2 11
Urutau Ponds 2 11
Meadows Stream 2 3
Surukua 1 1
Urutau Lagoon 1 1
Urutau Streams 0 0
Table 3 - Variation in vocalization activity across the study area; where species diversity refers to the number species recorded at a site over the entire study, and activity refers to the number of distinct times any of the seven study species were encountered at each site

Figure 3: The activity of each species, expressed as the number of distinct sightings, in the forest and meadow
Figure 4: The activity of each species expressed as the number of distinct sightings within each of the ten study sites.
Nocturnal variation of activity across habitat types
Anuran calls were recorded within every time zone in both forest and meadow sites. Below the changes in total anuran activity throughout the night are summarized between the two major habitat types (Forest and Meadow) in Figure 5 and further broken down for each study site in Table 4.

Figure 5: The overall change in activity throughout the night for forest sites and meadows sites
1800-2000 2000-2200 2200-0000 0000-0200 0200-0400
WG 1 2 3 2 3 1
WG2 3 4 3 5 3
WGS 1 1 0 1 0
AL1 5 5 4 4 2
AL2 3 3 3 3 2
Urutau Stream 0 0 0 0 0
Urutau Lagoon 1 0 0 0 0
Urutau Ponds 6 2 0 0 0
Tuca Pacova 7 5 5 4 4
Surukua 1 0 0 0 0
Table 4: The total activity at each site within each time zone.
Nocturnal variation in activity for different anuran species
Below the level of calling activity for each study species against time is shown in Figure 6.

Figure 6: Changes in level of activity for each species throughout the night.
Environmental Variables
The temperature for each trip into the field was recorded immediately before fieldwork began at, using a thermometer located at the field base. Temperature ranged between 8ºc and 24ºc and averaged at 16.8 ºc for all 150 sample periods. The average temperature at which each species is found active at is shown below in Table 5. Figures 7 (a) - (d) respectively show the relation between the activity of O. americanus, P. albonotatus, H. albopunctatus and H. caingua and the temperatures taken at the sites at which they were found to be present. No graphs are provided for R. ornata, R. schneideri, and Scinax berthae due to small sample size.
Temperature
|
| |
Minimum |
Maximum |
Mean |
| R. ornata |
20 |
24 |
22.7 |
| R. schneideri |
24 |
24 |
24 |
| H. albopunctatus |
21 |
24 |
23.2 |
| H. caingua |
8 |
24 |
17.8 |
| S. berthae |
21 |
21 |
21 |
| O. americanus |
13 |
24 |
20.5 |
| P. albonotatus |
16 |
24 |
20.3 |

Figures 7 (a), (b), (c) and (d) - The relationship between temperature and activity for O. americanus, P. albonotatus, H. albopunctatus and H. caingua respectively. In this case, activity refers to the percentage of samples, that each species was found to be active within for a given temperature. For each species, sites that had yielded no activity whatsoever were excluded from the data. (Click to enlarge)
Pitfall traps and casual observations
In total, 1854 individual trap checks were made between February 21st and May 18th and within them, 25 anurans were found giving a catch rate of 1.3%. In total seven species of anuran were found in the traps (four of which were not heard calling throughout the study period); R. ornata and R. schneideri (Familia Bufonidea), P. albonotatus and P. cuvieri (Familia Leiuperidae), Leptodactylus podicipinus and L. mystacinus (Familia Leptodactylidae) and Elachistocleis bicolor (Familia Microhylidae).
| Species |
Frequency |
Locations |
| R. ornata |
1 |
Surukua |
| R. schneideri |
1 |
Urutau |
| P. albonotatus |
1 |
Surukua |
| P. cuvieri |
15 |
Chachi, Surukua, Urutau Stream |
| E. bicolor |
1 |
Surukua |
| L .mystacinus |
2 |
Urutau Stream, Surukua |
| L. podicipinus |
4 |
Urutau Stream, Surukua |
Table 6 - Pitfall trapping catch frequency and location for anuran species.
The catch rate between sites varied between 0% at Urutau Lagoon to 2.1% at the Surukua trail, but was too low to show significant comparisons between any of the sites.
In addition, the following species were also identified through casual observations throughout the study period: Dendropsophus minutus, Dendropsophus nanus, Hypsiboas raniceps and Scinax fuscovarius (Familia Hylidae).
DISCUSSION
The habitat use of calling species
For the majority of the study species, calling frogs showed no dependence on any particular microhabitat. The one exception, H. albopunctus was only ever heard calling at one pond (site 7, see Figures 2 and 4). This species was discovered calling at this site on six out of the fifteen occasions (40%) it was visited. Given that it was heard at no other study site, it is likely that this pond is an important breeding site for this species within the local area. Why H. albopunctatus was only found breeding at this pond requires further study but it is possible that the physical characteristics of this pond, including its size, depth and surrounding vegetation may have provided some benefit to this species.
The distribution of breeding activity was far more wide-ranging for some of the other study species. In fact, four species (R. ornata, O. americanus, H. caingua, and P. albonotatus) were recorded within both the forest and the surrounding meadows (Figure 3). All of these four species were recorded calling at at least three out of the ten study sites and in the case of H. caingua seven sites yielded breeding activity (Figure 4). For these species no significant difference can be shown between their activities among forest or meadow sites and it seems likely that pond characteristics have a greater effect on calling behaviour than the surrounding habitat
R. schneideri and S. berthae were each heard calling at only one forest site (Sites 3 and 5 respectively, see Figures 2 and 4). The low sample size (both species were only heard calling on one occasion throughout the entire study) makes it impossible to make conclusions about the importance of these sites for the breeding of the species.
It is important to underline that results about habitat use presented above in Figures 3 and 4 and in Table 3 do not necessarily represent habitat preferences for these species. The results simply reflect detection rate based on vocal activity of the species, which may tend to identify breeding sites over habitats used for toher purposes. In fact, adult anurans commonly spend most of their lives in terrestrial habitats (Wilbur 1984, cited by Marsh and Trenham), and it would be wrong to make conclusions about the range of various populations based on data collected from a few isolated ponds.
Calling activity across different time zones
Overall anuran activity within the forest peaked between 1800 and 2000 and decreased throughout the night (see Figure 5 and Table 4). No such pattern was exhibited among the meadow sites where activity fluctuated randomly throughout the night (see Figure 5) and peaked at 16 recordings in both 2000-2200 and 0000-0200. The effects of time on the activity on each individual species were also recorded. Interestingly all species were found active between 1800 and 2000, and all but H. caingua showed a decrease in activity after this point (see figure 5). On the contrary activity of H. caingua increased throughout the night and peaked between 0000 and 0200 before decreasing again (see figure 6). More research is required to identify the reasons why calling activity peaked between 1800 and 2000 for the majority of species, but it may be affected by temperature.
Anuran activity against temperature
Of the four species that provided a sufficient amount of data, three (H. albopunctatus, P. albonotatus and O. americanus) exhibited activity that correlated strongly with temperature, see Figures 7 (a), (b) and (c). The activities of these three species seem to show a relationship with both time and temperature. Regression analysis needs to be completed to show which, if either, of these factors may be affecting calling activity. Again H.caingua did not fit the typical pattern with temperature having no correlation with the calling behaviour of the species (see Figure 7d).
Existence of non-calling individuals
Unfortunately the number of specimens caught through pitfall trapping was too low to make comparisons or meaningful conclusions about the separate sites. However, through pitfall trapping and personal observations nine further species were found within the study area, which were not recorded calling throughout the study period. Whilst this does not eliminate the possibility that the breeding areas of these species were overlooked during the study, the failure to record vocal activity in these species may also be interpreted as them not breeding during the time of the study (ie seasonal differentiation in breeding). If the latter is true this suggests that using calling behaviour, though efficient, is not enough to monitor the entire anuran fauna at any given time and must be used in conjunction with other sampling tecnhiques in order to better understand the anuran diversity of any given area. Moreover, these additional observations provided useful information on some of the calling species. For example, C. schneideri though only recorded calling once in the study period, and in a forest habitat, was abundant in grassland habitats and around the houses at the field base. Furthermore S. berthae which too was only heard calling once throughout the study (at a site in the forest), and had previously never been found within SRNP was later caught by Pier Cacialli in the meadows.
Project Improvements and Future directions
The above notes provide information on the existence of species found in PROCOSARA for only a small part of the year, and where and when they have so far been shown to be found for at least a part of their life cycle. In order to create a fuller and more realistic picture of the distribution of anuran species within the area several guidelines and improvements are suggested for future researchers:
A more refined measure for activity
The measure of activity should be refined by estimating the number of calling males found at each study site. Results from the current study only show whether a species was present or absent at a given site. A more flexible approach would provide a larger data set and would undoubtedly provide a far more in-depth set of results, e.g. how variables such as temperature, time or even interspecific competition affect activity, effect the activity of each species.
Increase distance between study sites
As the movement of anurans between breeding ponds is common (Spieler and Linsemair, 1997), its probable, that given the low distances between the study sites used, that some dispersal between sites occurred throughout the study period. This may have resulted in the double-counting of some individuals.
Monitor diurnal activity
Though most anurans are regarded as having a largely nocturnal activity, throughout the duration of the project a number of individuals, notably, P. albonotatus and O. americanus were heard calling during the day, beginning as early as 1230. Diurnal calling evidently does exist, and should not be ignored. A complete study of the temporal activity of anurans should ideally be recorded throughout the course of the 24 hour day.
Increased capture of individuals to ensure correct species identity
Though using pre-recorded samples to compare individuals is essentially good practice, future researchers should be aware that (i) intraspecific variation in calling behaviour will likely exist across and possibly even within different populations and (ii) distinct but similar species may (though certainly not always) have very similar calls and (iii) anuran taxonomy is in a state of flux with new species being described and species being split with increasingly regularity. Capture and collection of calling individuals is the safest way to reach a correct identification both at the time of the study and for accurately comparing results with future studies. In the case of this study, H. caingua and H. pulchella had seemingly similar calls according to Stranek (1995). Both species were found within PROCOSARA, though only calling individuals of H. caingua were captured. After listening to H. caingua there was a reasonable amount of variation in the calls and it is possible, though never confirmed, that H. pulchella could have accounted for some of this activity. In the case of these two species, even a sighting may not be enough to tell them part. After investigating samples at the natural history museum in Asuncion the only discernible difference between the species were the spots on the dorsal part of the forearm of H. pulchella.
Increased use of pitfall traps and inclusion of drift fences and other methodologies
Monitoring calling behaviour of anurans reveals their presence during only a small portion of a given species’ life cycle. As adult anurans spend the majority of their life in terrestrial habitats the need to monitor this activity is vital to gain a true understanding of community structure. The continued and greater use of pitfall traps should be used alongside other methodologies such as using drift fences or active searches where a specific number of logs (for example) are overturned within a given time period. In this study the use of pitfall traps was confined to the forests, as traps placed in meadows or marshes either flooded or were deemed a hazard to the livestock kept in these areas. It is hoped that future studies may be able to use designated areas of the meadows for pitfall trapping as it is obvious that they yield high anuran activity.
Creation of a monitoring database of anuran activity throughout the year
The short term objective of this report was to create a preliminary list of the species present in the study area, their preferred habitats and time budgets of activity levels during breeding. It is hoped that this data will give future interns a head start about where, how and when to study anurans at San Rafael, and that its conclusions will also help future interns create a project plan that will more effectively monitor anuran activity. In the long term, it is hoped that research will be continued to be carried out in the area throughout the course of the year and that a database for the activity (using a standardised measure) for all found species in the property can be regularly logged and updated continuously throughout the year. If this is achieved, in time hopefully patterns will begin to emerge showing which seasons, climatic conditions and habitat types are most strongly associated with each species. Eventually it may even be possible in the very long term to monitor how these activities change from year to year allowing estimations to be made on the status of the anuran fauna within SRNP, ultimately allowing us to make better efforts to protect them.
APPENDIX
Annotated ist of the 16 amphibian species recorded during the study
Family Bufonidae
Rhinella ornata (Spix, 1824). Though only recorded calling on three occasions, the volume and frequency of calling suggested that individuals were aggregating en masse. This was confirmed at site 10, next to stream leaving the large lagoon, where huge numbers of toads possibly exceeding a total of 100 were found breeding.
Rhinella schneideri (Werner, 1894). Much the most conspicuous and abundant species of anuran recorded by sight, regularly found in and around human dwellings.
Family Hylidae
Dendropsophus minutus (Peters, 1872). Captured in areas between sites 8 and 9 but never recorded calling
Dendropsophus nanus (Boulenger, 1889)
Hypsiboas albopunctatus (Spix 1824)
Hypsiboas caingua (Carrizo, 1991)
Hypsiboas pulchellus (Duméril & Bibron, 1841)
Hypsiboas raniceps (Cope, 1862) Commonly seen around the large lagoon and near human dwellings but never recorded calling.
Scinax fuscovarius (Lutz, 1925). Also commonly found in human dwellings, particularly in bathrooms.
Scinax berthae. Previously never found within SRNP. A call, nearly exactly the same to that provided by Stranek (1995) was recorded by a small pond on the Tuca Pacova trail. The suspicion was later confirmed when an individual was captured by Pier Cacialli in the meadows between sites 8 and 9.
Familia Cicloramphidae
Odonotophrynus americanus (Duméril & Bibron, 1841). Frequently heard calling during the day in both the forest and meadows.
Familia Leptodactylidae
Leptodactylus mystacinus (Burmeister, 1861)
Leptodactylus podicipinus (Cope, 1862)
Familia Leiuperidae
Physalaemus albonotatus (Steindachner, 1864)
Physalaemus cuvieri (Fitzenger, 1826)
Familia Microhylidae
Elachistocleis bicolor (Guérin-Méneville, 1838). Captured in a pitfall trap on the Surukua trail after huge thunderstorms had caused many of the traps to flood.
REFERENCES
Alford RA, Richards SJ 1999 - Global amphibian declines: a problem in applied ecology - Annual Review of Ecology Systematics 30: p133-165.
Barboza F, Pinazzo J, Fracchia F - 1997 - Bosque Atlántico Interior 1997. Mapa. Proyecto Sistema Ambiental de la Región Oriental (SARO). Asunción: Ministerio de Agricultura y Ganadería y World Wildlife Fund.
Barinaga M 1990 - Where have all the Froggies gone? - Science 247: p1033-1034
Berven KA 1990 - Factors affecting population fluctuations in larval and adult stages of the wood frog (Rana sylvatica) Ecology 71: p1599-1608
Esquivel AM, Velazquez MC, Bodrati A, Fraga R, Del Castillo H, Klavins J, Madroño A, Perris SJ 2007 - Status of the avifauna of San Rafael National Park, one of the last large segments of Antlantic Forest in Paraguay - Bird Conservation International 17: p301-317
Eterovick PC, Carnaval ACOQ, Borges-Nojosa DM, Silvano DL, Sazima I - 2005 Amphibian declines in Brazil: an overview - Biotropica 37: p166-179
Fisher RN, Schaffer HB 1996 - The decline of amphibians in California’s Great Central Valley - Conservation Biology 10: 1387-1397.`
Fragano F, Clay RP 2003 - Biodiversity Status of the Interior Atlantic Forest of Paraguay. In: Galindo-Leal, Camara The Atlantic Forest of South America - Island Press, Washington, USA.
Gardener T, Fitzherbert E, Hill N, Williams H 2001 - An ecological research project concerned with the assessment and monitoring of anuran populations in the region around Las Cuevas, Chiquibul Forest Reserve, Belize.
Heyer WR, Rand AS, Cruz CAG, Peixoto OL 1988 - Decimations, extinctions, and colonisations of frog populations in southeast Brazil and their evolutionary implications - Biotropica 20: p230-235.
Laurance WF, McDonald KR, Speare R 1996 - Epidemic disease and the catastrophic decline of Australian rain forest frogs - Conservation Biology 10: p400-413.
Luddeke H 2001 - Male and female responses to call playback in the Andean frog Colostethus subpunctatus.
Marsh DM, Trenham PC 2000 Metapopulation dynamics and amphibian conservation - Conservation Biology 15: p40-49.
Motte M, Nuñez K 2002 - Anfibios y Reptiles. p55-62 en SEAM, CDC & MNHNP. Evaluación Ecológica Rápida: Reserva San Rafael. Proyecto PAR/94/001/PNUD/DINCAP/MAG. Asunción, Paraguay.
Myers N, Mitter-Meier RA, Fonseca GAB, Kent J 2000 - Biodiversity hotspots for conservation priorites. Nature 403: 853-858.
Pounds JA, Crump ML 1994 - Amphibian declines and climate disturbance: The case of the Golden Toad and the Harlequin Frog - Conservation Biology 8: p72 - 85.
Spieler M, Linsenmair KE 1997 - Choice of optimal oviposition sites by Hoplobatrachus occipitalis (Anura: Ranidae) in an unpredictable and patchy environment - Oecologia 109: p184-199
Stranek, R., de Olmedo, E. V., Carrizo, G. R. (1993): Catalogo de voces de anfibios argentinos Parte 1 - LOLA, Buenos Aires.
Toledo LF, Haddad CFB 2005 - Acoustic Repertoire and Calling Behaviour of Scinax fuscomarginatus (Anura, Hylidae) - Journal of Herpetology 39: p455-464.








