

Designed by Paul Smith 2006. This website is copyrighted by law.
Material contained herewith may not be used without the prior written permission of FAUNA Paraguay.
Photographs on this web-site were taken by Paul Smith, Hemme Batjes, Regis Nossent, Frank Fragano,
Alberto Esquivel, Arne Lesterhuis, José Luis Cartes, Rebecca Zarza and Hugo del Castillo and are used with their permission.
Priodontes maximus (Kerr 1792) Image Gallery
TAX: Class Mammalia; Subclass Theria; Infraclass Eutheria; Order Cingulata; Family Dasypodidae; Subfamily Tolypeutinae, Tribe Priodontini (Myers et al 2006, Möller-Krull et al 2007). The genus Priodontes was described by F. Cuvier (1825). Formerly placed in its own subfamily Priodontinae, Möller-Krull et al (2007) provided DNA evidence that demonstrated their position within the Tolypeutinae. E.Geoffroy St-Hilaire´s (1803) description was based on specimen "No CCCCXIV" with reference to de Azara´s (1801) "Le Grand Tatou". G.Cuvier´s (1817) description was based on an illustration of "Le Kabassou" in Buffon (1763), who wrote that it was the largest tatou and came from Cayenne, the type locality. Larrañaga´s (1923) description was based on de Azara´s "Maximo". Synonyms adapted from Gardner (2007):
Dasypus maximus Kerr 1792:112. Type locality "Cayenne", French Guiana.
Dasypus giganteus É. Geoffroy St-Hilaire 1803:207. Type locality "Le Paraguay" referenced to de Azara (1801). Restricted to Pirayú, Departamento Paraguarí by Cabrera (1958).
Dasypus gigas G.Cuvier 1817:221. Based on Buffon (1763).
D[asypus]. gigans Schmid 1818:164. No type locality.
T[atus] grandis Olfers 1818:219. Type locality "Paraguay".
Priodontes giganteus Lesson 1827:309. Name combination.
D[asypus]. (P[riodontes].) Gigas Voigt 1831:261. Name combination.
Priodonta gigas Gray 1843:120. Name combination.
Priodon gigas Owen 1845:21. Name combination.
Prionodontes gigas Schinz 1845:316. Name combination.
Prionodos gigas Gray 1865:374. Name combination.
Prionodon gigas Gray 1869:380. Name combination.
Cheloniscus gigas Fitzinger 1871:227. Name combination.
Priodontes maximus O.Thomas 1880:402. First use of current name.
Priodon maximus JA Allen 1895:187. Name combination.
D[asypus]. maximus Larrañaga 1923:343. Type locality "Nemoribus septentrionalibus paraquarensibus". Based on de Azara (1802).
ENG: Giant Armadillo (Gardner 2007).
ESP: Tatú gigante (Emmons 1999), Armadillo gigante (Esquivel 2001).
GUA: Tatu carreta (Neris et al 2002, Villalba & Yanosky 2000), Kry´y pura vachu Ac (Esquivel 2001), Krypuravachú Ac (Villalba & Yanosky 2000), Tatu guazu MA (Emmons 1999, Villalba & Yanosky 2000), Nambirope A (Villalba & Yanosky 2000), Jautare P (Villalba & Yanosky 2000), Tatú-wasu (Chebez 1996), Tatu-carrera (Redford & Eisenberg 1992 - transcription error??). Tatú carreta is the most commonly-used named for the species in Paraguay and is used in preference to the Spanish names.
DES: A huge armadillo, most easily recognised by its size and flat carapace which does not cover the sides of the body. Carapace flattened and largely hairless, with rectangular scales aligned in rows. Colouration dark greyish centrally with pale, buffy border - though pattern sometimes obscured by earth from excavations. There are 11 to 13 movable dorsal bands on the carapace, making it extremely flexible and 3 to 4 movable neck bands. The head is relatively small., pale-coloured and with well-separated ears split by armoured scales. The head shield is oval-shaped and not expanded between the eyes. Muzzle rounded and somewhat conical, blunt at the end with a small mouth opening. Tail long, covered with small, pale, pentagonal scales and narrowing towards the tip. Underside naked, lacking either armour or hair, and with a pinkish-brown colouration. Feet are large, especially the hind feet and the X toes of the forefeet each bear a large, scimitar-shaped claw, the third being particularly long (up to 20.3cm). Females have two teats. Individuals can be distinguished by scale patterm, particularly the dividing line between the dark and light scales on the carapace and hind legs and the number of light scales per row from the lower edge of the carapace up to the dividing line (Noss et al 2004). CR: No information. DF: Armadillos lack true teeth, but possess a series of "molariform" teeth that do not follow the standard mammal dental formula. Dentition in this species is abundant but poorly differentiated and teeth are shed with age. As a result the number of teeth reported is highly variable. An individual captured in Argentina for example had 65 teeth. 20-25/20-25 = 80-100. CN: 2n=50.
TRA: Distinctive foreprint like a broken scythe-shape with a broad, long "comma" situated towards the outer part of the print separated from an oval-shaped impression on the inner part of the print. Hind print with four well-formed, round-tipped toes, the outer two toes somewhat smaller than the inner two, and the innermost slightly separated from the rest. With the exception of the long third toe, the print is notably wider than it is long. A large oval pad leaves an impression below toe 3. The heavy tail leaves a zig-zag shaped scratch mark between the prints, formed by the rolling gait of the animal. FP: 5.5 x 3.4cm HP: 4.9 x 3.8cm. (Villalba & Yanosky 2000). Faeces typically have a flat surface and measure 14.7 (+/-1.7mm) x 22.7mm (+/-3mm). They are of firm consistency and have a weak acrid odour, containing up to 85% soil and almost no plant material (0.1%). Remains of ants and termites prominent. Weight 2.3g (+/-0.8mm). Typically found near areas of excavation and at burrow entrances (Anacleto 2007).
MMT: Much the largest armadillo in Paraguay, more than twice the size and five times the weight of the next largest species Euphractus sexcinctus. TL: (147-160cm); HB: 89.5cm (75-100cm); TA: 52.82cm (48-60cm); FT: 19.1cm (18-20cm); EA: 5.38cm (4.5-6cm); WT: 26.8kg (18.7-45kg) Captive individuals may reach 80kg in weight; WN: 113g. (Parera 2002, Nowak 2001, Ceresoli et al 2003, Emmons 1999, Redford & Eisenberg 1992).
SSP: Identifiable by size alone, adults are unmistakable on account of their great bulk. Juveniles potentially confusable with Naked-tailed Armadillos of the genus Cabassous, which share the greatly developed claws on the forefeet, but even adults of that genus are notably smaller than juvenile Giant Armadillos. Note also that the carapace of the Giant Armadillo seems to rest on the back, whereas that of other armadillos covers the sides and flanks. Furthermore Naked-tailed Armadillos have, as the name suggests, greatly-reduced scaling on the tail.
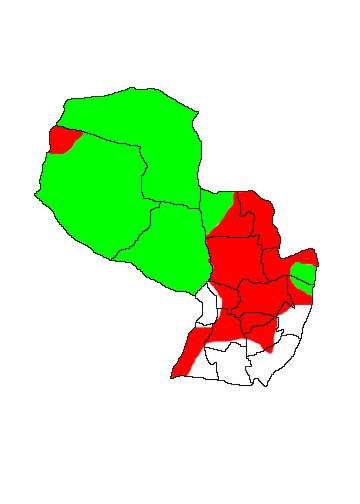
DIS: Widely distributed at low density in South America east of the Andes. Occurs from southeast Venezuela south through the Amazon Basin of Colombia, Ecuador, Brazil, Peru and Bolivia through Paraguay to northern Argentina. It is absent from eastern Brazil and there are no records from Uruguay or Chile. Historically probably occurred to about 31ºS in Argentina (corresponding to Provincia Córdoba and Santa Fé) but today it is confined to Formosa, Chaco, Salta and northern Santiago del Estero (Parera 2002). Records from Misiones and Corrientes are considered doubtful as the name "Tatu carreta" is also applied to Cabassous tatouay there (Chebez 1996) and there are no specimens from either province. In Paraguay it has disappeared over much of its former range and is extinct over vast swathes of the Orient. Small and possibly unsustainable populations remain in the larger Itaipú Reserves in Departamento Alto Paraná, in the Mbaracayú Forest Reserve in Departamento Candindeyú (though there are no recent records) and in more remote areas of Departamento Concepción. There are no records from Departamento Misiones or Central (though the species probably occurred historically at least in the latter) and only a single record in Itapúa and the species is now extinct there as in much of the rest of eastern Paraguay. It remains widespread in the Chaco but local extinctions occur where human populations are established and it has apparently disappeared from the Nueva Asunción and PN Tte Enciso area, northern Departamento Boquerón (Neris et al 2002).
HAB: Found mainly in undisturbed forest, scrub and grassland, it rapidly disappears from inhabited areas. In the Central Chaco it most often encountered in areas xerophytic and semi-xerophytic areas vegetated with Lapacho (Tabebuia sp) and Palo Santo (Bulnesia sarmientoi) trees, but also occurs in deciduous forest in the Pantanal area and more open, seasonally-flooded palm savanna in the Humid Chaco. During a camera-trapping survey it was not recorded in the dry Chaco alluvial plains of Santa Cruz Bolivia, though it was present where there is forest cover, however the authors noted that animals occurring at very low density could easily escape attention (Noss et al 2004). In eastern Paraguay it formerly occurred at the edge of humid forest and cerrado savanna, but has disappeared from the vast majority of its former range and is in decline wherever it remains. Merritt (2006) note the preferred habitat of the species in the Paraguayan Chaco to be riparian areas or areas with loose, sandy-loam soil. The presence of substantial food reserves appears to be vital for the existence of the species and affinity for areas near water has also been noted (Nowak 1991). Habitat choice is apparently unaffected by fire, a study in the cerrado of Mato Grosso, Brazil finding that they utilised burnt areas as frequently as they do unburnt areas when foraging (Prada & Marinho-Filho 2004). Burning is of course a natural occurrence in the cerrado biome and does not directly affect the species main prey items.
ALI: Almost entirely myrmecophagous, feeding for the most part on ants (especially Atta sp) and termites with the addition of other subterranean invertebrates found in their colonies. Anacleto (2007) studied the diet of the species in the cerrado of Mato Grosso, Brazil and found only Hymenoptera and Isoptera in the faeces. Ants made up 27.5% of the biomass of the faeces, with the termite Cornitermes accounting for 60.6%. Other termite species including Velocitermes, Nasutitermes and Coptotermes made up just 0.9% of the biomass. The nest of Cornitermes is the most resistant to opening, requiring considerable force to break it open, but termites with more resistant nests have weaker chemical defences. Giant Armadillo foraging strategy is very similar to that of anteaters in that it uses the massive claws of the forefeet to break open nests and then feeds on the occupants. However one major difference is that the armadillo frequently destroys the colony at a single feeding necessitating a roaming rather than a territorial existence. Neris et al (2002) state that animals in the Paraguayan Chaco feed mainly on the larvae and honey of ground nesting bees. Carrion and vertebrates such as snakes have also been reported in the diet of the species, but these would seem to be unusual occurrences (Parera 2002). Captive individuals have been maintained on meat and meat-based formulas (Merritt 2006).
REP: Litter size is one or two (usually one), born after a gestation period of four months in a burrow dug by the mother (Redford & Eisenberg 1992, Neris et al 2002). Young have tough, leathery skin and are weaned at 4 to 6 months. Sexual maturity is reached at 10 to 12 months (Neris et al 2002, Parera 2002, Nowak 1991).
BEH: General Behaviour Solitary, nocturnal or crepuscular and highly fossorial and so rarely observed. Noss et al (2004) found the species to be most active between 10pm and 6am in Santa Cruz, Bolivia. Individuals pass the daylight hours in large burrows (40-45cm wide x 30-31cm high) dug with their powerful front claws and often built into sandbanks, or occasionally active or dead termite mounds. Carter & Encarnaçao (1983) found the mean dimensions of burrows to be 45cm wide x 32cm high at ground level, increasing to 47x34cm 10cm inside the burrow, much larger than any other armadillo species and instantly recognisable by size alone. Typically the upper part of the burrow entrance is pointed rather than rounded (Emmons 1999), the mean burrow slope is 34.4º and they are dug in a direction so that the prevailing wind blows away from the entrance (Carter & Encarnaçao 1983). When digging the adult may pose on its hind legs and tail and throw the whole wait of its body behind violent strikes with the forelegs, the massive claws able to inflict considerable damage onto even hard-baked soil and termite nests. After the initial "attack" the loosened soil is scraped using the forefeet towards the hind feet where it is then kicked behind the body. (Emmons 1999). Burrows are often clumped and inhabited for at least 24 hours, though frequently a single burrow may be occupied for several nights (Redford & Eisenberg 1992) and one female occupied the same hole for 17 consecutive days (Carter & Encarnaçao 1983). In Brazil 68% of burrows were located in open grassy habitats, 28% in areas prone to flooding and just 3% in forested habitats and almost half of these were in active termite mounds (Nowak 1991). The open country burrows were located an average of 192m from forest edge. Adults leave their burrow only to feed and to look for a mate. They are surprisingly capable swimmers. Though the species is not territorial, the minimum home range has been estimated at an average of 452.5ha and individuals may cover 3km in a single night (mean 2,765m) (Parera 2002, Carter 1985). According to camera trap data home ranges of males overlap considerably (Noss et al 2004) and the maximum distance moved by a single individual was given as 7.5km. Published life spans of the species are between 12 to 15 years. This species walks on the soles of the hindfeet and only the tips of the claws of the forefeet are in contact with the ground (Vizcaíno & Milne 2002). Defensive Behaviour The species has a well-developed sense of smell but poor eyesight, and on the approach of a potential threat they rise up onto the hind legs, supported by the tail and begin to sniff from side to side. This position is similar to the defensive position adopted by anteaters and enables them to strike out with their sharply-hooked claws if suddenly attacked. For the most part their usual response to danger is to flee or to begin to dig rapidly into the substrate, digging in with the claws so that they cannot be dislodged. Enemies Besides man Giant Armadillos probably count on very few natural enemies. Their low density and large size means that they would not form a substantial part of the diet of any predator. However there are records of the species being attacked by Jaguar in Venezuela and Puma might also be expected to occasionally prey on the species.
VOC: No information.
HUM: Attractive to hunters on account of its large size and eyecatchingly large and often clumped burrows which are frequently re-used, allowing the occupant to be "staked out". The meat is considered "invigorating", whilst the fat is used for the treatment of asthma and bronchitis (Neris et al 2002). The rarity and large size of the species means that trophy hunting is as big a threat as hunting for the table (Villalba & Yanosky 2000). The species has almost disappeared from eastern Paraguay and occurs at naturally low densities in the Chaco which means that it is not likely to be hunted in large numbers, however individuals unlucky enough to stray close to populated areas put themselves at great risk. Locals state that it possesses an acute sense of hearing that enable it to detect the presence of predators from a considerable distance (Neris et al 2002). The species is frequently accused of digging up vegetables, but it is likely that this is a side-effect of digging for insects (Nowak 1991).
CON: The Giant Armadillo is considered Vulnerable by the IUCN, click here to see their latest assessment of the species. The Centro de Datos de Conservación in Paraguay consider the species to be in imminent danger of extinction in Paraguay and give it the code N1. The species is listed on CITES Appendix 1 - click here to see the justification. The species occurs at naturally low density on account of its nomadic feeding behaviour, but does not tolerate the close presence of humans. Estimates of its decline vary widely and population figures for large areas of intact habitat do not exist. Over its entire range it is estimated to have declined by 30% over the last 20 to 30 years by the IUCN, whereas a decline of 50% in the last decade has also been noted. It has a low fecundity rate and populations rapidly fall victim to hunters. Adults require large home ranges with adequate food supplies and so local extinction has occurred over much of eastern Paraguay, though its secretive habits mean that it is undoubtedly under-recorded in the Chaco. Minimum home range has been estimated at an average of 452.5ha in Argentina (Parera 2002, Carter 1985), whilst crude estimates of 5-8 individuals per 100km2 were made for the species in Santa Cruz, Bolivia from camera trap data. Assuming that all specimens remained in the area in which they were photographed they estimated a maximum of 14 individuals per 100km2 for the Tucavaca region of Santa Cruz, Bolivia, though they noted that the majority of animals were photographed only once during the 28 month study (Noss et al 2004). The population remains healthy and relatively well-protected in the Chaco as a result of the isolation of the habitat, but it is at risk from conversion of natural habitat into ranchland and desertification in areas of extensive deforestation. Burning can have a severe impact on the species, with 2 individuals being found burnt to death in a 2000ha area following a fire in Emas National Park. Intensive pesticide use in agricultural areas actively eliminates their food source. Historically it has been considered a zoological "rarity" and was much sought after for zoological gardens and private collections. Vulnerability to fire would seem to be correlated to the intensity of the blaze (in turn related to the combustability of the vegetation), and regular burning may in fact be less damaging to the species than infrequent but more severe fires. Burning does not apparently affect the species choice of habitat, it being equally as frequent in burned areas as unburnt areas in the cerrado of Brazil (Prada & Marinho-Filho 2004). Any conservation strategy should be accompanied an educational element in which local people are taught to admire and respect the species and myths about its supposed great value on the black market are dispelled (Porini 2001). A captive breeding programme is currently underway in Argentina with the aim to reintroduce the species and augment wild populations.
Citable Reference: Smith P (2007) FAUNA Paraguay Online Handbook of Paraguayan Fauna Mammal Species Account 6 Priodontes maximus.
Last Updated: 30 January 2009.
References:
Allen JA 1895 - On the Names Given by Kerr in his "Animal Kingdom" Published in 1792 - Bulletin AMNH 7: p179-192.
Anacleto TC de S 2007 - Food Habits of Four Armadillo Species in the Cerrado Area, Mato Grosso, Brazil - Zoological Studies 46: p529-537.
Azara F de 1801 - Essais sur l´Histoire Naturelle des Quadrupèdes de la Province du Paraguay - Charles Pougens, Paris.
Azara F de 1802 - Apuntamientos para la Historia Natural de los Quadrúpedos del Paraguay y Rio de la Plata - La Imprenta de la Viuda de Ibarra, Madrid.
Buffon GL le Clerc 1763 - Histoire Naturelle, Générale et Particulière, avec la Description du Cabinet du Roi - L´Imprimerie Royale, Paris.
Cabrera A 1958 - Catálogo de los Mamíferos de América del Sur - Revista Museo Aregntino de Ciencias Naturales Bernadino Rivadavia Zoology 4: p1-307.
Carter TS, Encarnaçao CD 1983- Characteristics and Use of Burrows by Four Species of Armadillos in Brazil - Journal of Mammalogy 64: p103-108.
Cartés JL 2007 - Patrones de Uso de los Mamíferos del Paraguay: Importancia Sociocultural y Económica p167-186 in Biodiversidad del Paraguay: Una Aproximación a sus Realidades - Fundación Moises Bertoni, Asunción.
Ceresoli N, Jimenez GT, Duque EF 2003 - Datos Morfómetricos de los Armadillos del Complejo Ecológico de Sánz Peña, Provincia del Chaco, Argentina - Edentata 5: p35-37.
Chebez JC 1996 - Fauna Misionera - LOLA, Buenos Aires.
Cuvier G 1817 - Le Règne Animal Distribué d´après son Organisation, pour Servir de Base a l´Histoire Naturelle des Animaux et d´Introduction à l´Anatomie Comparée - Deterville, Paris.
de la Peña MR, Pensiero JF 2004 - Plantas Argentinas: Catálogo de Nombres Comunes - LOLA, Buenos Aires.
Emmons LH 1999 - Mamíferos de los Bosques Húmedos de América Tropical - Editorial FAN, Santa Cruz.
Esquivel E 2001 - Mamíferos de la Reserva Natural del Bosque Mbaracayú, Paraguay - Fundación Moises Bertoni, Asunción.
Fitzinger LJ 1871 - Die Natürliche Familie der Gürtheliere (Dasypodes) I Abtheilung - Sitzungsber. Kaiserl. Akad. Wiss. Wien 64:p209-276.
Gardner AL 2007 - Mammals of South America Vol 1: Marsupials, Xenarthrans, Shrews and Bats - University of Chicago Press, Chicago.
Geoffroy St-Hilaire É 1803 - Catalogue des Mannifères du Muséum National d´Histoire Naturelle - Paris.
Gray JE 1843 - List of the Specimens of Mammalia in the Collection of the British Museum - British Museum of Natural History, London.
Gray JE 1865 - Revision of the Genera and Species of Entomophagous Edentata, Founded on the Examination of Specimens in the British Museum - Proceedings of the Zoological Society of London 1865: p359-386.
Gray JE 1869 - Catalogue of Carnivorous, Pachydermatous and Edentate Mammalia in the British Museum - British Museum of Natural History, London.
Jones ML 1982 - Longeivty of Captive Mammals - Zool. Garten 52: p113-128.
Kerr R 1792 - The Animal Kingdom or Zoological System of the Celebrated Sir Charles Linnaeus. Class I. Mammalia: Containing a Complete Systematic Description, Arrangement and Nomenclature of all the Known Species and Varieties of the Mammalia, or Animals which give Suck to their Young; Being a Translation of that Part of the Systema Naturae as Lately Published with Great Improvements by Professor Gmelin of Goettingen. Together with Numerous Additions from more Recent Zoological Writers and Illustrated with Copperplates - A.Strahan, T Cadell & W Creech, Edinburgh.
Larrañaga A 1923 - Escritos - Instituto Històrico y Geogràfico del Uruguay 2: p1-512.
Lesson RP 1827 - Manuel de Mammalogie ou Histoire Naturelle des Mammifères - Roret, Paris.
Merritt DA 2006 - Research Questions on the Behavior and Ecology of the Giant Armadillo (Priodontes maximus) - Edentata 7: p23-25.
Möller-Krull M, Delsuc F, Churakov G, Marker C, Superina M, Brosius J, Douzery EJP, Schmitz J 2007 - Retroposed Elements and Their Flanking Regions Resolve the Evolutionary History of Xenarthran Mammals (Armadillos, Anteaters and Sloths) - Molecular Biology and Evolution 24: p2573-2582.
Myers P, Espinosa R, Parr CS, Jones T, Hammond GS, Dewey A 2006 - The Animal Diversity Web (online). Accessed December 2007.
Neris N, Colman F, Ovelar E, Sukigara N, Ishii N 2002 - Guía de Mamíferos Medianos y Grandes del Paraguay: Distribución, Tendencia Poblacional y Utilización - SEAM, Asunción.
Noss AJ, Peña R, Rumiz DI 2004 - Camera Trapping Priodontes maximus in the Dry Forests of Santa Cruz, Bolivia - Endangered Species Update 21: p43-52.
Nowak RM 1991 - Walker´s Mammals of the World 5th Ed Volume 1 - Johns Hopkins, Baltimore.
Olfers I 1818 - Bemerkungen zu Illiger´s Ueberblick der Säugthiere, nach Ihrer Vertheilung über die Welttheile, Rücksichtlich der Südamerikanischen Arten p192-237 in Bertuch FI Neue Bibliothek der Wichtigsten Reisebeschreibungen zue Erweiterung der Erd - und Völkerkunde; in Verbindung mit Einigen Anderen Gelehrten Gesammelt und Herausgegeben - Verlage des Landes-Industrie-Comptoirs, Weimar.
Owen R 1845 - Odontography or a Treatise on the Comparative Anatomy of the Teeth; their Physiological Relations, Mode of Development, and Microscopic Structure in the Vertebrate Animals - Hippolyte Bailiere, London.
Parera A 2002 - Los Mamíferos de la Argentina y la Región Austral de Sudamérica - Editorial El Ateneo, Buenos Aires.
Porini G 2001 - Tatu Carreta Priodontes maximus en Argentina - Edentata 4: p9-14.
Prada M, Marinho-Filho J 2004 - Effects of Fire on Abundance of Xenarthrans in Mato Grosso, Brazil - Austral Ecology 29: p568-573.
Redford KH, Eisenberg JF 1992 - Mammals of the Neotropics: Volume 2 The Southern Cone - University of Chigaco Press, Chicago.
Schinz HR 1845 - Systematisches Verzeichniss aller bis jetzt Bekannten Säugthiere oder Synopsis Mammalium nach dem Cuvier´schen System 2 - Jent und Gassmann, Solothurn.
Schmid K 1818 - Naturhistorische Beschreibung der Säugthiere - Verlage der Lithographischen Kunst-Anstalt, München.
Thomas O 1880 - On Mammals from Ecuador - Proceedings of Zoological Society of London 1880: p393-403.
Villalba R, Yanosky A 2000 - Guía de Huellas y Señales: Fauna Paraguaya - Fundación Moises Bertoni, Asunción.
Vizcaíno SF, Milne N 2002 - Structure and Function in Armadillo Limbs (Mammalia: Xenarthra: Dasypodidae) - Journal of Zoological Society of London 257: p117-127.
Voight FS 1831 - Das Thierreich, Geordnet nach seiner Organisation, als Grundlage der Naturgechichte der Thier und Einleitung in die Vergleichende Anatomie von Baron von Cuvier. Erster Band, Säugthiere und Vögel - FA Brockhaus, Leipzig.
ACKNOWLEDGEMENTS
Many thanks to Mariella Superina for assisting with obtaining some of the references used in the construction of this species account.
MAP 6: Priodontes maximus


